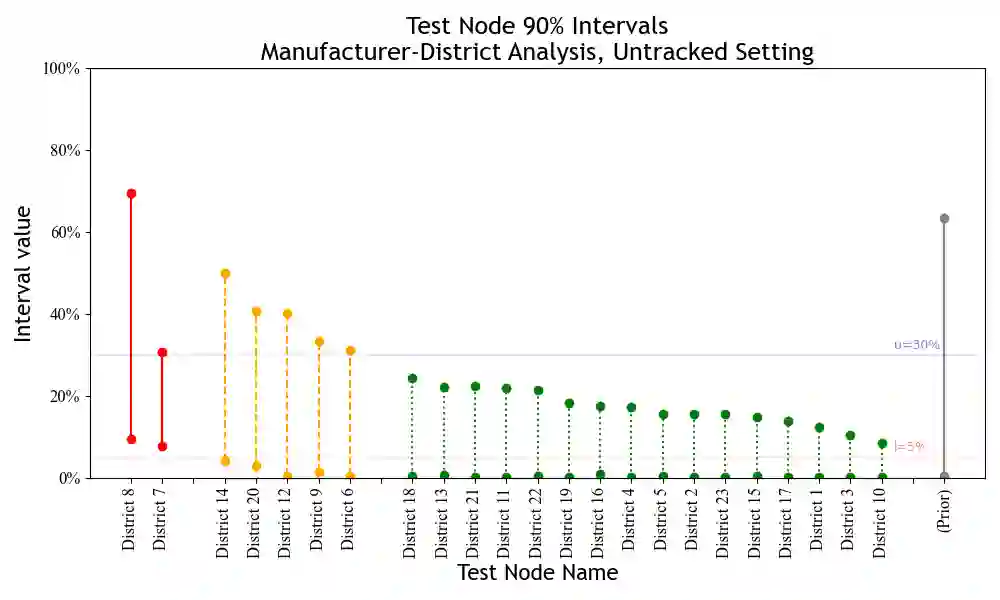
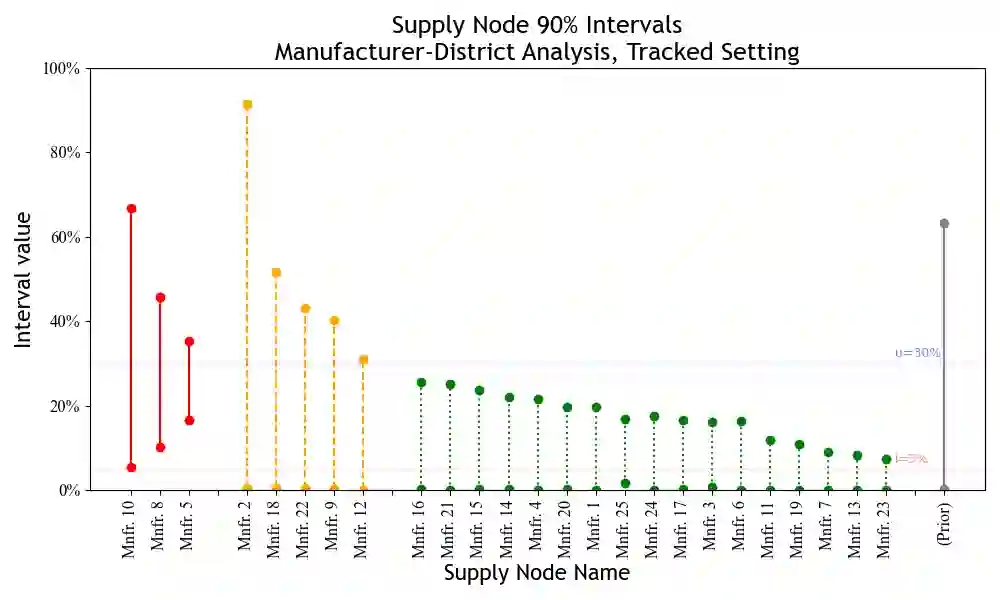
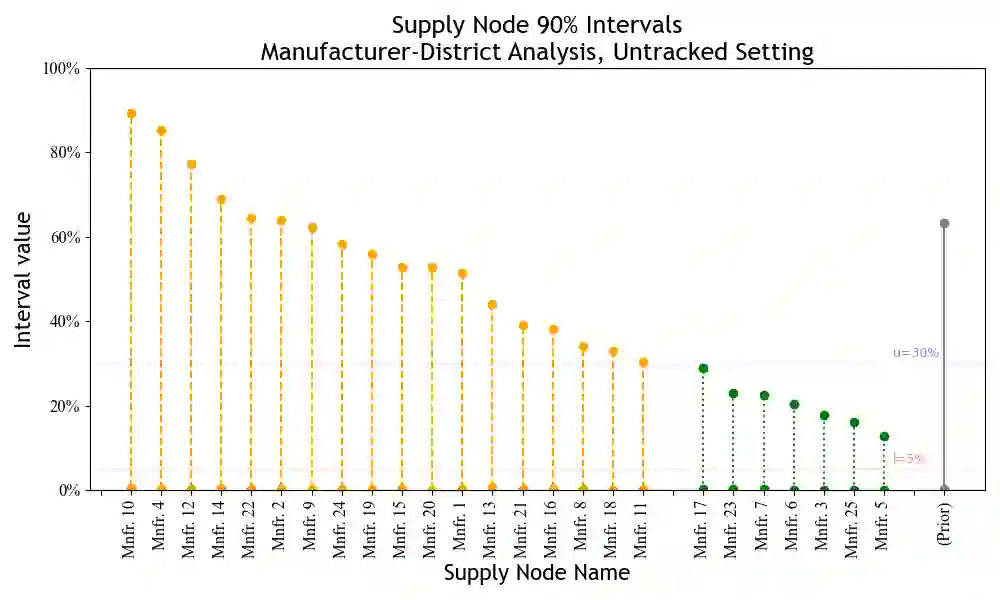
Substandard and falsified pharmaceuticals, prevalent in low- and middle-income countries, substantially increase levels of morbidity, mortality and drug resistance. Regulatory agencies combat this problem using post-market surveillance by collecting and testing samples where consumers purchase products. Existing analysis tools for post-market surveillance data focus attention on the locations of positive samples. This paper looks to expand such analysis through underutilized supply-chain information to provide inference on sources of substandard and falsified products. We first establish the presence of unidentifiability issues when integrating this supply-chain information with surveillance data. We then develop a Bayesian methodology for evaluating substandard and falsified sources that extracts utility from supply-chain information and mitigates unidentifiability while accounting for multiple sources of uncertainty. Using de-identified surveillance data, we show the proposed methodology to be effective in providing valuable inference.
翻译:低中收入国家盛行的低于标准和伪造药品大幅度提高了发病率、死亡率和抗药性水平。监管机构通过在消费者购买产品的地方收集和测试市场后监测样品来解决这一问题。现有的市场后监测数据分析工具将注意力集中在积极样品的地点。本文件希望通过利用不足的供应链信息来扩大这种分析,以提供低于标准和伪造产品的来源的推断。我们首先确定在将这种供应链信息与监测数据相结合时存在无法识别的问题。然后,我们制定贝叶西亚方法,评估低于标准和伪造的来源,从供应链信息中提取有用性,减少不可识别性,同时核算多种不确定来源。我们利用非固定的监测数据,表明拟议方法在提供有价值的推断方面是有效的。