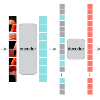
Dose volume histogram (DVH) metrics are widely accepted evaluation criteria in the clinic. However, incorporating these metrics into deep learning dose prediction models is challenging due to their non-convexity and non-differentiability. We propose a novel moment-based loss function for predicting 3D dose distribution for the challenging conventional lung intensity modulated radiation therapy (IMRT) plans. The moment-based loss function is convex and differentiable and can easily incorporate DVH metrics in any deep learning framework without computational overhead. The moments can also be customized to reflect the clinical priorities in 3D dose prediction. For instance, using high-order moments allows better prediction in high-dose areas for serial structures. We used a large dataset of 360 conventional lung patients with 2Gy $\times$ 30 fractions to train the deep learning (DL) model using clinically treated plans. We trained a UNet-like CNN architecture using computed tomography (CT), planning target volume (PTV) and organ-at-risk contours (OAR) as input to infer corresponding voxel-wise 3D dose distribution. We evaluated three different loss functions: (1) Mean Absolute Error (MAE) Loss, (2) MAE + DVH Loss, and (3) the proposed MAE + Moments Loss. The quality of the predictions was compared using different DVH metrics as well as dose-score and DVH-score, recently introduced by the AAPM knowledge-based planning grand challenge. Model with (MAE + Moment) loss function outperformed the model with MAE loss by significantly improving the DVH-score (11%, p$<$0.01) while having similar computational cost. It also outperformed the model trained with (MAE+DVH) by significantly improving the computational cost (48%) and the DVH-score (8%, p$<$0.01). The code, models, docker container, and Google Colab project are available on our DoseRTX GitHub (https://github.com/nadeemlab/DoseRTX).
翻译:Dose 音量直方图(DVH) 是诊所广泛接受的评估标准。 但是,将这些计量纳入深度学习剂量预测模型中具有挑战性。 我们提出一个新的基于时空的损失函数, 用于预测具有挑战性的常规肺密度调制辐射治疗(IMRT)计划3D剂量分布。 基于时空的损失函数具有共和性和差异性, 可以很容易地将DVH指标纳入任何深层次学习框架, 而不计算间接费用。 时间也可以被定制, 在 3D 剂量预测中反映临床优先事项 。 例如, 使用高调的OHD 数据模型可以在高剂量区域对序列结构进行更好的预测 。 我们使用一个大型的数据集, 使用 2Gy $ 30 分数的临床处理计划来培训深度学习( DL ) 模型。 我们用数学模型( 、 规划目标量( PTVMA) 和 组织- 风险模型( OAR ) 用于对匹配的 Vox- 质量预测值 3OH 进行快速分析, 模型( MA 3) 成本分配: 不同的模型, 不同的模型( 不同的模型, 不同的模型, 不同的模型, 以MA- d) 不同的模型( 不同的模型, 不同的模型, 和 以不同的磁号) 改进了。