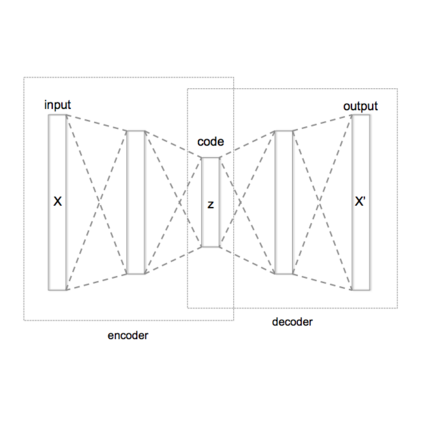
Shape information is a strong and valuable prior in segmenting organs in medical images. However, most current deep learning based segmentation algorithms have not taken shape information into consideration, which can lead to bias towards texture. We aim at modeling shape explicitly and using it to help medical image segmentation. Previous methods proposed Variational Autoencoder (VAE) based models to learn the distribution of shape for a particular organ and used it to automatically evaluate the quality of a segmentation prediction by fitting it into the learned shape distribution. Based on which we aim at incorporating VAE into current segmentation pipelines. Specifically, we propose a new unsupervised domain adaptation pipeline based on a pseudo loss and a VAE reconstruction loss under a teacher-student learning paradigm. Both losses are optimized simultaneously and, in return, boost the segmentation task performance. Extensive experiments on three public Pancreas segmentation datasets as well as two in-house Pancreas segmentation datasets show consistent improvements with at least 2.8 points gain in the Dice score, demonstrating the effectiveness of our method in challenging unsupervised domain adaptation scenarios for medical image segmentation. We hope this work will advance shape analysis and geometric learning in medical imaging.
翻译:在医学图象中的分解器官之前,形状信息是十分有力和宝贵的。然而,目前大多数深层次的基于学习的分解算法没有将信息纳入考虑之中,这可能导致对纹理的偏向。我们的目标是以明确的形状为模型,并用它来帮助医学图像的分解。以前的方法提议了基于变式自动自动编码器(VAE)的模型,以学习特定器官的形状分布,并用它来自动评估分解预测的质量,将其与所学的分解分布相匹配。基于这个方法,我们的目标是将VAE纳入目前的分解管道。具体地说,我们提议在假损失和VAE重建损失的基础上建立一个新的不受监督的域适应管道。两种损失都是同时优化的,作为回报,还提高了分解任务性工作性。对三种公共分解数据集以及两个内部的Pancreas分解数据集的广泛实验表明,在Dice评分中至少增加了2.8个分数。我们的方法在挑战医学分解的地理图象的分解模型分析中,将展示我们的方法的有效性。