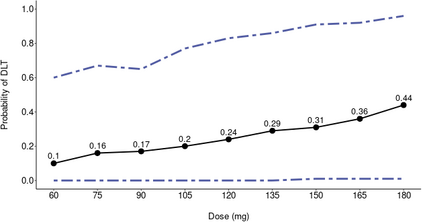
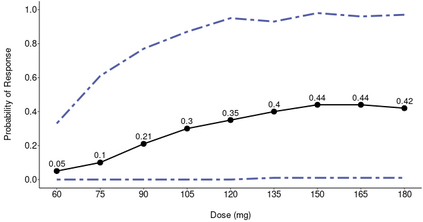
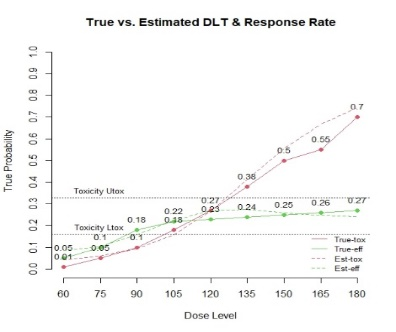
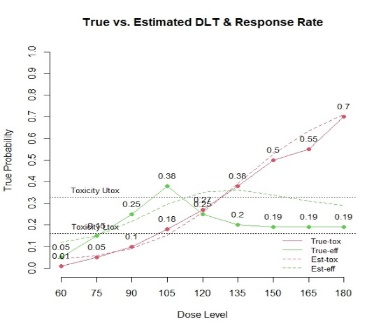
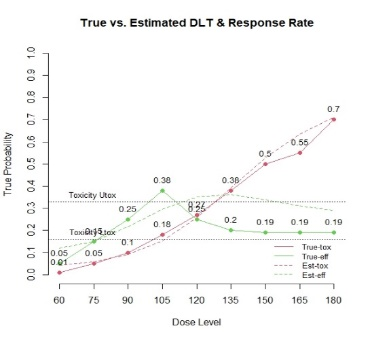
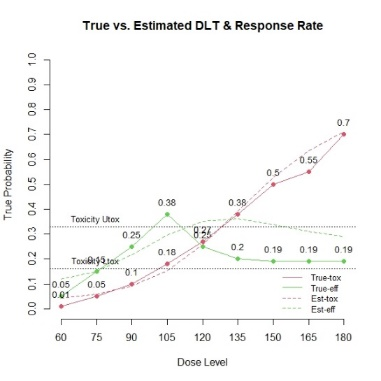
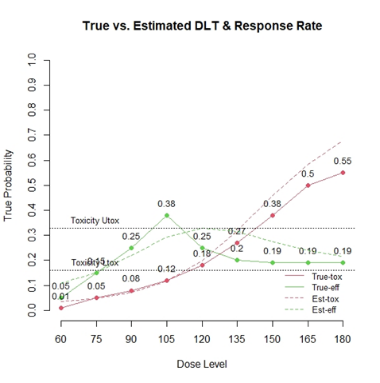
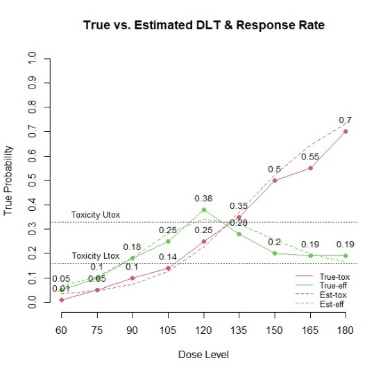
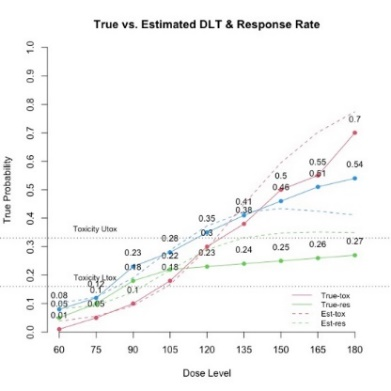
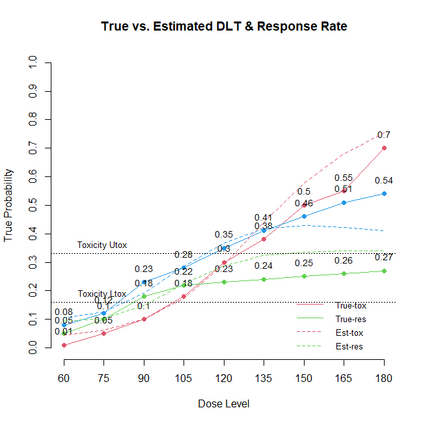
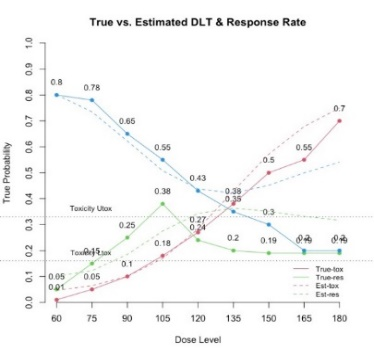
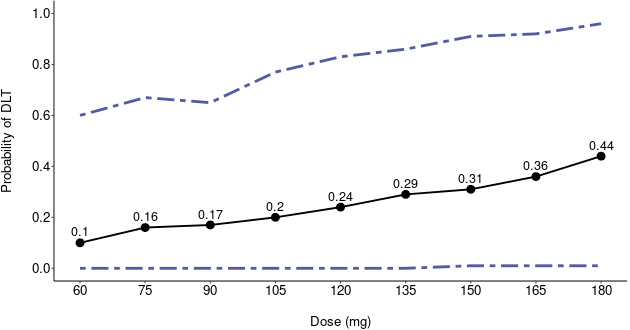
The primary objective of phase I oncology studies is to establish the safety profile of a new treatment and determine the maximum tolerated dose (MTD). This is motivated by the development of cytotoxic agents based on the underlying assumption that the higher the dose, the greater the likelihood of efficacy and toxicity. However, evidence from the recent development of cancer immunotherapies that aim to stimulate patients' immune systems to fight cancer challenges this assumption, particularly further escalation after a certain dose level might not necessarily increase the efficacy. Dose escalation study of molecular targeted agents (MTA) often does not only rely on the safety profile. In this paper, we propose a simple and flexible model that uses multivariate Gaussian latent variables to integrate toxicity endpoint and efficacy biomarker. This model can be easily extended to incorporate additional immune biomarkers. By simultaneously considering multiple outcomes, the proposed method is better at identifying the biologically optimal dose, which results in better decision-making. Simulation studies showed that the proposed method has desirable operating characteristics by determining the target dose with an optimal risk-benefit trade-off. We have also implemented our proposed method in a user-friendly R Shiny tool.
翻译:第一阶段肿瘤学研究的首要目标是确定新治疗的安全状况并确定最大容许剂量(MTD),其动机是发展细胞毒性物剂,其基本假设是剂量越高,效力和毒性的可能性就越大。然而,最近发展旨在刺激病人免疫系统以对抗癌症的癌症免疫疗法对这个假设提出了挑战,特别是在一定剂量水平后进一步升级不一定提高效果。分子目标物剂的剂量升级研究往往不只依靠安全特征。在本文件中,我们提出了一个简单灵活的模型,使用多变高斯潜伏变量将毒性终点和功效生物标记结合起来。这一模型可以很容易地推广到包括更多的免疫生物标记。在考虑多种结果的同时,拟议方法能够更好地确定生物最佳剂量,从而更好地决策。模拟研究表明,拟议的方法具有理想的操作特征,通过以最佳风险-效益交易确定目标剂量。我们还在用户友好型的R Shiny工具中采用了我们提出的方法。