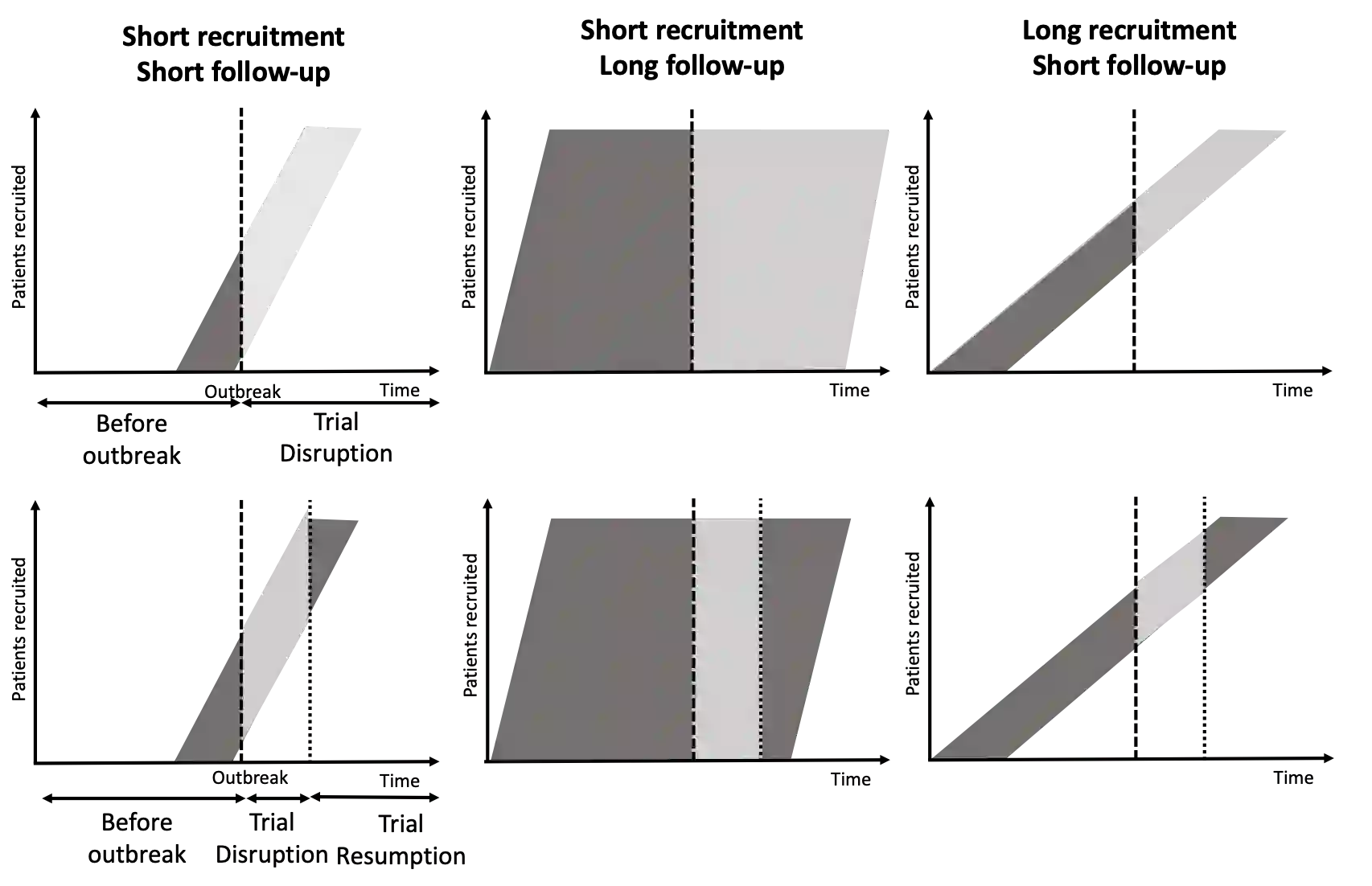
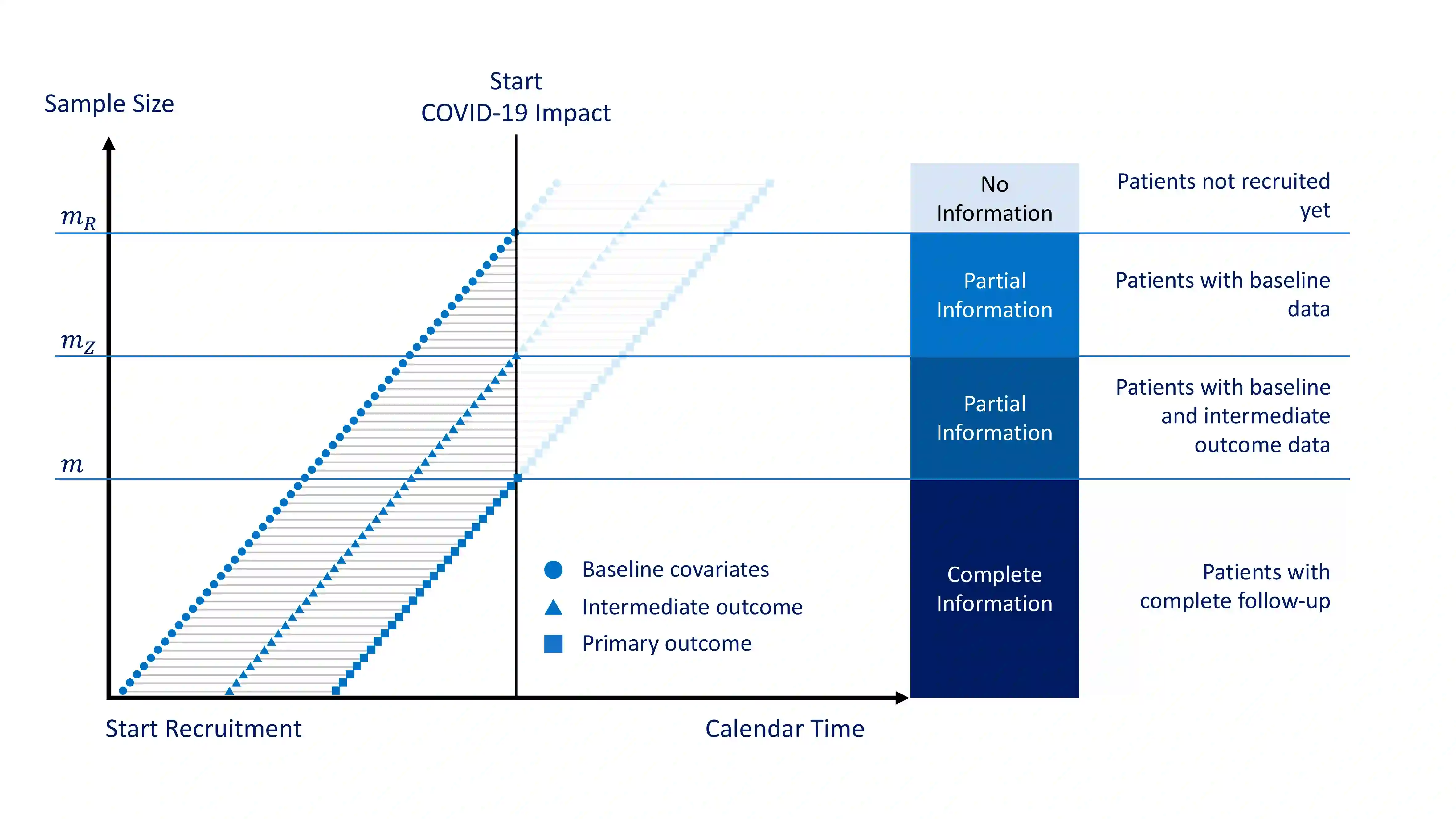
Clinical trials disruption has always represented a non negligible part of the ending of interventional studies. While the SARS-CoV-2 (COVID-19) pandemic has led to an impressive and unprecedented initiation of clinical research, it has also led to considerable disruption of clinical trials in other disease areas, with around 80% of non-COVID-19 trials stopped or interrupted during the pandemic. In many cases the disrupted trials will not have the planned statistical power necessary to yield interpretable results. This paper describes methods to compensate for the information loss arising from trial disruptions by incorporating additional information available from auxiliary data sources. The methods described include the use of auxiliary data on baseline and early outcome data available from the trial itself and frequentist and Bayesian approaches for the incorporation of information from external data sources. The methods are illustrated by application to the analysis of artificial data based on the Primary care pediatrics Learning Activity Nutrition (PLAN) study, a clinical trial assessing a diet and exercise intervention for overweight children, that was affected by the COVID-19 pandemic. We show how all of the methods proposed lead to an increase in precision relative to use of complete case data only.
翻译:虽然SARS-COV-2(COVID-19)大流行导致临床研究的启动令人印象深刻和史无前例,但也导致其他疾病地区的临床试验严重中断,在这种流行病期间,约80%的非COVID-19试验停止或中断,在许多情况下,中断的试验将不具备产生可解释结果所必需的计划统计能力,本文件介绍了通过纳入辅助数据来源提供的其他信息来弥补因试验中断而造成的信息损失的方法,所述方法包括使用试验本身提供的基线和早期结果数据的辅助数据,以及采用常客和巴耶斯方法纳入外部数据来源的信息,这些方法通过应用基于初级保健儿科学习活动营养研究的人工数据分析、评估受COVID-19大流行影响的超重儿童饮食和练习干预的临床试验,说明所有拟议方法如何提高仅使用完整案例数据的精确度。