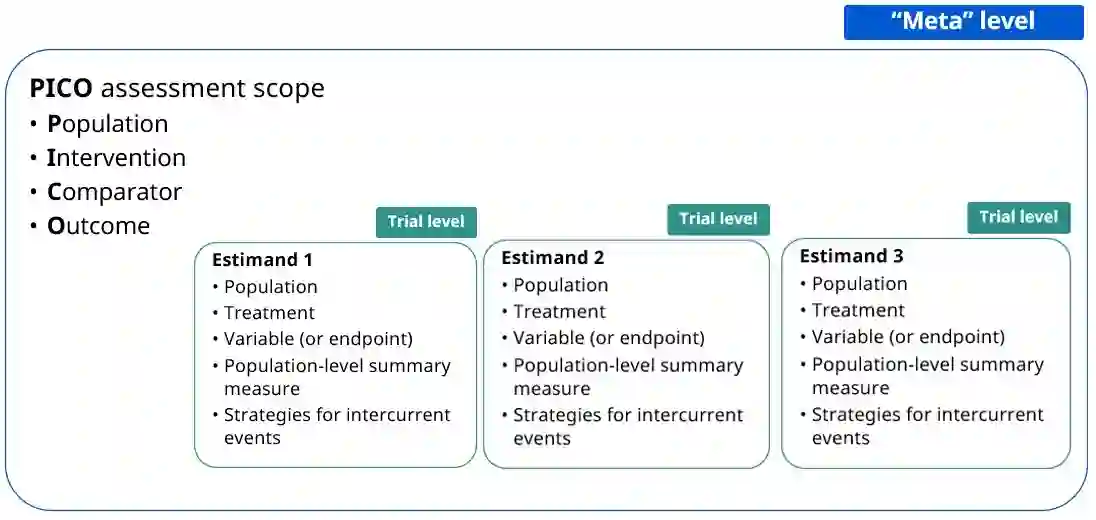
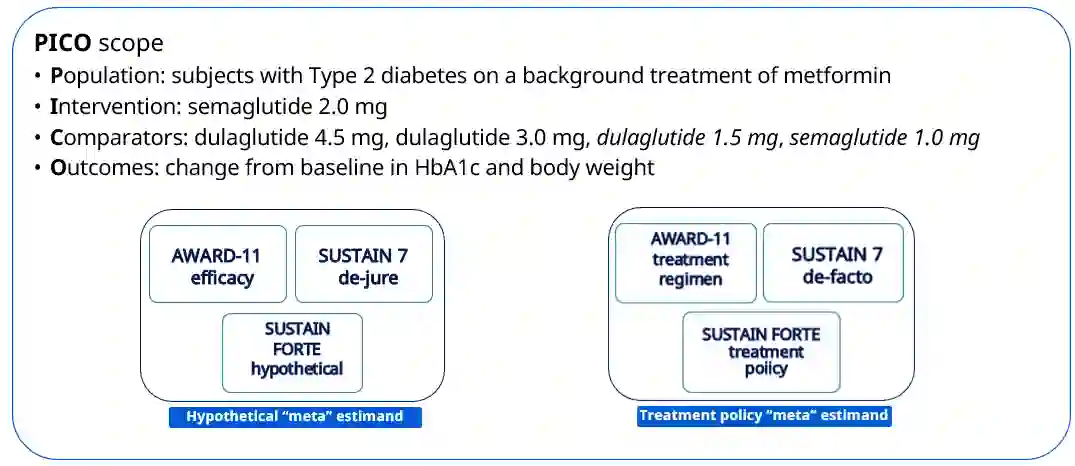
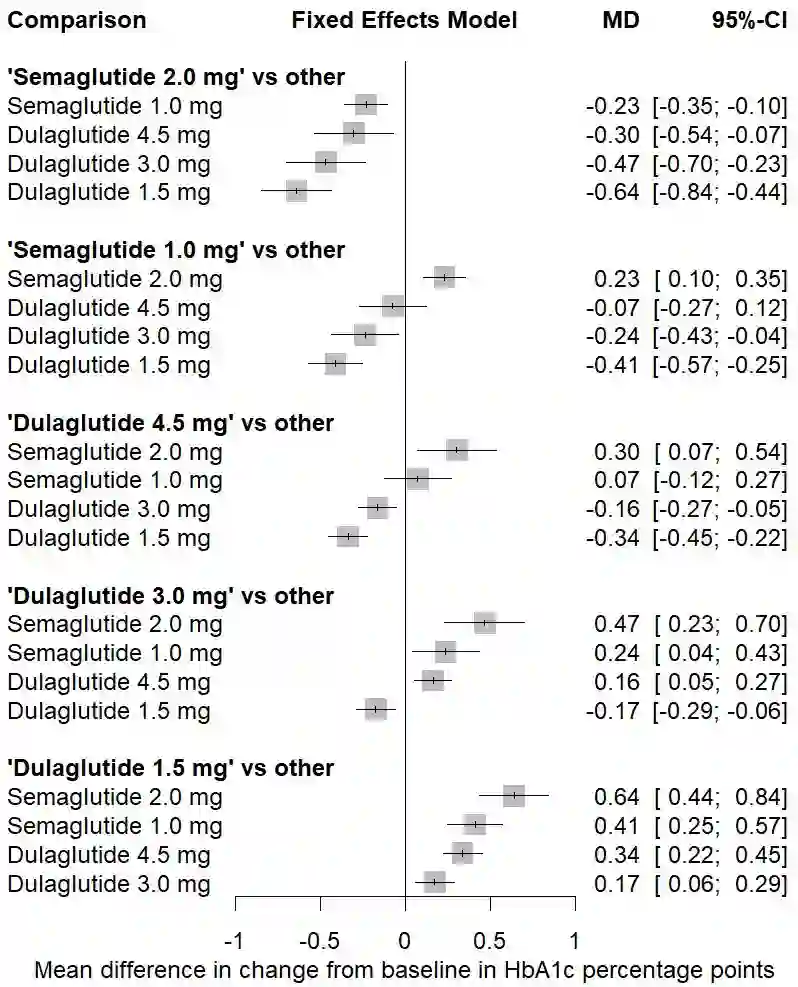
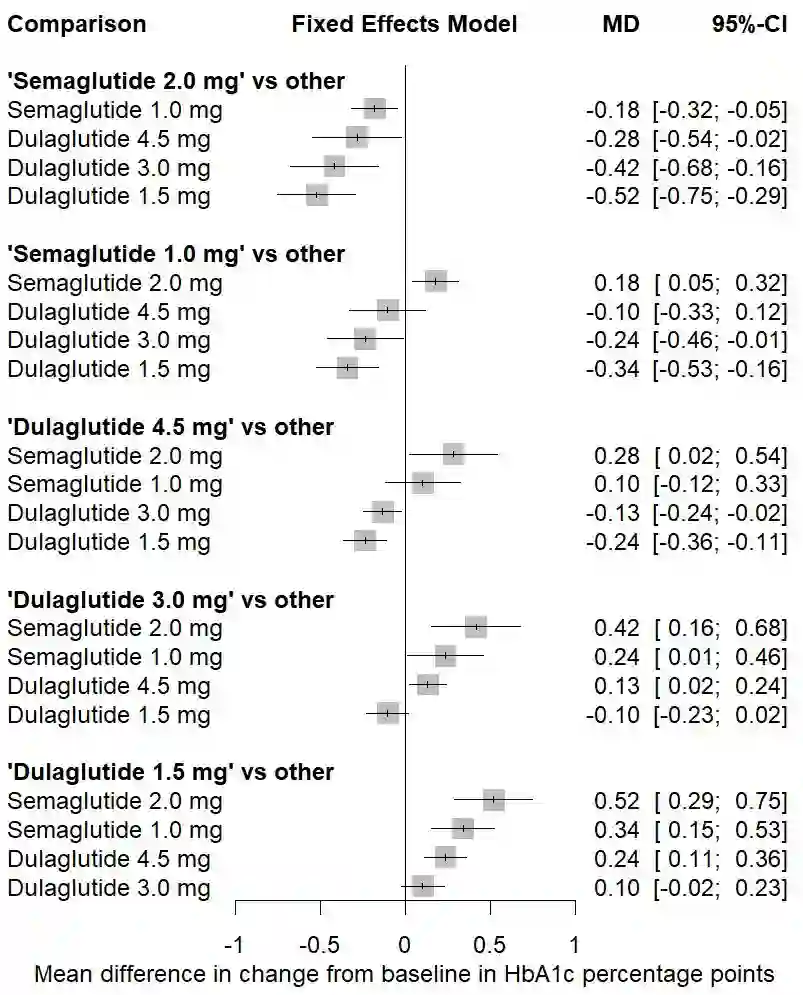
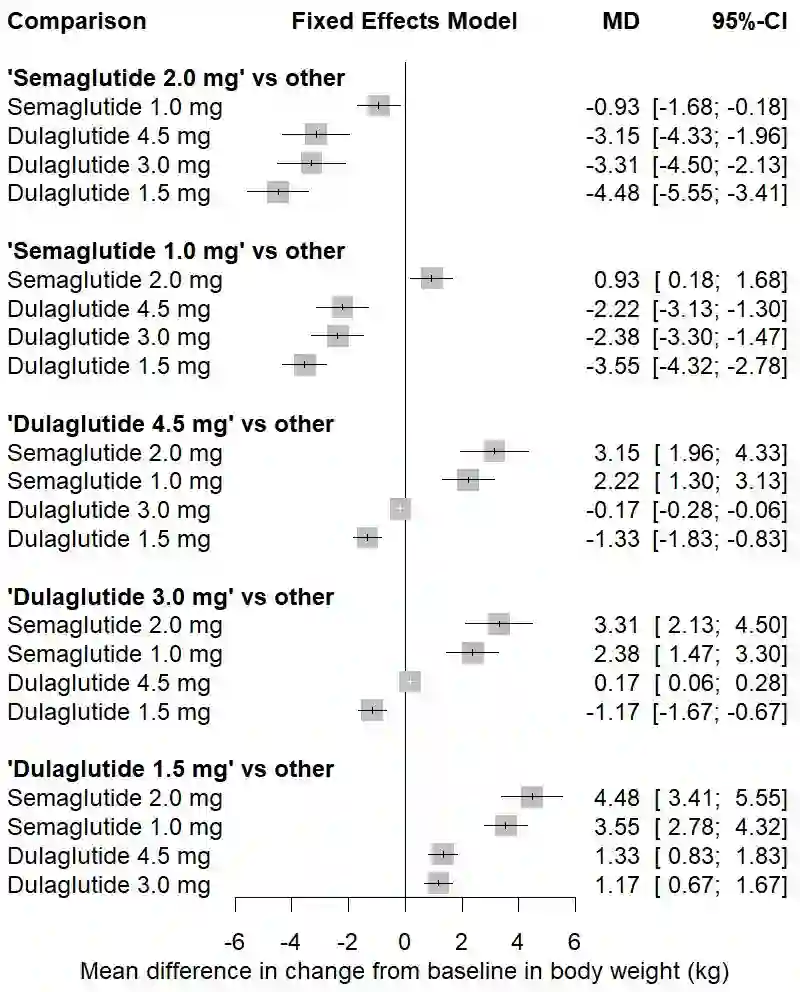
The estimand framework is increasingly established to pose research questions in confirmatory clinical trials. In evidence synthesis, the uptake of estimands has been modest, and the PICO (Population, Intervention, Comparator, Outcome) framework is more often applied. While PICOs and estimands have overlapping elements, the estimand framework explicitly considers different strategies for intercurrent events. We propose a pragmatic framework for the use of estimands in meta-analyses of clinical trials, highlighting the value of estimands to systematically identify and mitigate key sources of quantitative heterogeneity, and to enhance the applicability or external validity of pooled estimates. Focus is placed on the role of strategies for intercurrent events, within the specific context of meta-analyses for health technology assessment. We apply the estimand framework to a network meta-analysis of clinical trials, comparing the efficacy of semaglutide versus dulaglutide in type 2 diabetes. We explore the impact of a treatment policy strategy for treatment discontinuation or initiation of rescue medication versus a hypothetical strategy for the corresponding intercurrent events. The specification of different target estimands at the meta-analytical level allows us to be explicit about the source of heterogeneity, the intercurrent event strategy, driving any potential differences in results. We advocate for the integration of estimands into the planning of meta-analyses, while acknowledging that potential challenges exist in the absence of subject-level data. Estimands can complement PICOs to strengthen communication between stakeholders about what evidence syntheses seek to demonstrate, and to ensure that the generated evidence is maximally relevant to healthcare decision-makers.
翻译:在确证性临床试验中,估计目标框架正日益成为提出研究问题的既定方法。在证据综合领域,估计目标框架的应用尚不广泛,而PICO(人群、干预、对照、结局)框架则更为常用。尽管PICO与估计目标框架存在重叠要素,但估计目标框架明确考虑了处理并发事件的不同策略。我们提出一个实用的框架,用于在临床试验的荟萃分析中应用估计目标,强调估计目标在系统性识别和减少定量异质性关键来源方面的价值,以及其在提升合并估计值的适用性或外部效度方面的作用。本文重点关注在卫生技术评估的荟萃分析这一特定背景下,处理并发事件的策略所扮演的角色。我们将估计目标框架应用于一项临床试验的网络荟萃分析,比较司美格鲁肽与度拉糖肽在2型糖尿病中的疗效。我们探讨了针对治疗中止或启用急救药物这两种并发事件,采用治疗策略策略与假设性策略所产生的影响。在荟萃分析层面明确不同的目标估计量,使我们能够清晰揭示驱动结果潜在差异的异质性来源——即并发事件处理策略。我们主张将估计目标整合到荟萃分析的规划中,同时承认在缺乏个体水平数据的情况下可能存在挑战。估计目标可作为PICO框架的补充,以加强利益相关方之间关于证据综合旨在证明内容的沟通,并确保生成的证据与医疗决策者的需求高度相关。