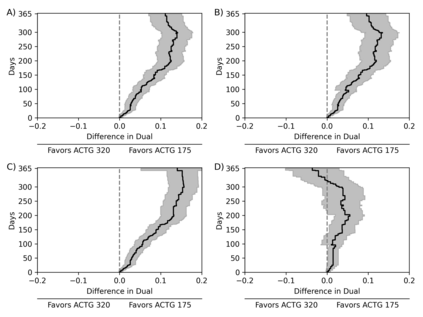
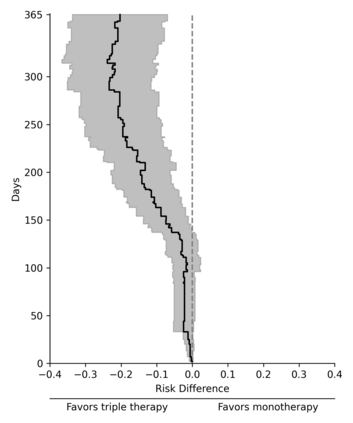
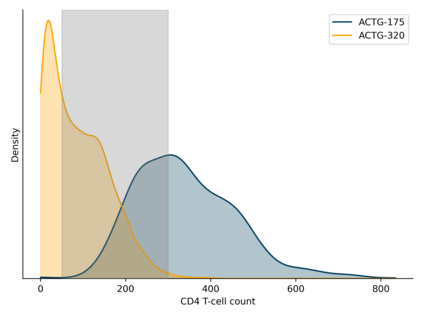
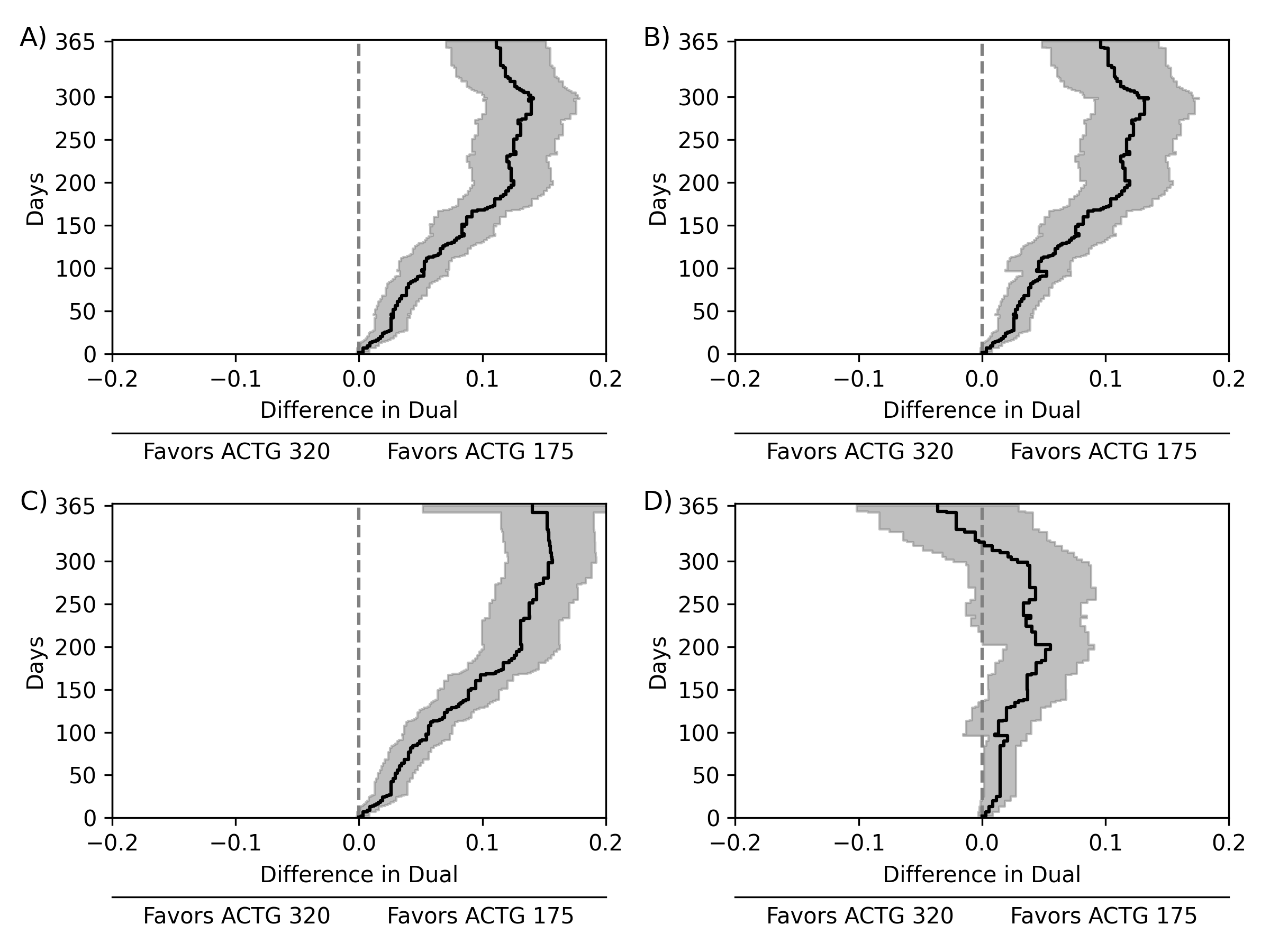
Comparisons of treatments or exposures are of central interest in epidemiology, but direct comparisons are not always possible due to practical or ethical reasons. Here, we detail a data fusion approach that allows bridged treatment comparisons across studies. The motivating example entails comparing the risk of the composite outcome of death, AIDS, or greater than a 50% CD4 cell count decline in people with HIV when assigned triple versus mono antiretroviral therapy, using data from the AIDS Clinical Trial Group (ACTG) 175 (mono versus dual therapy) and ACTG 320 (dual versus triple therapy). We review a set of identification assumptions and estimate the risk difference using an inverse probability weighting estimator that leverages the shared trial arms (dual therapy). A fusion diagnostic based on comparing the shared arms is proposed that may indicate violation of the identification assumptions. Application of the data fusion estimator and diagnostic to the ACTG trials indicates triple therapy results in a reduction in risk compared to mono therapy in individuals with baseline CD4 counts between 50 and 300 cells/mm$^3$. Bridged treatment comparisons address questions that none of the constituent data sources could address alone, but valid fusion-based inference requires careful consideration.
翻译:对治疗或接触的比较是流行病学的中心利益,但由于实际或伦理原因,直接比较并非总有可能实现。这里,我们详细介绍了一种数据混合法,使各研究之间能够进行过渡性治疗比较。激励性实例涉及比较在分配三重治疗和单一抗逆转录病毒疗法时艾滋病毒感染者综合结果的风险,即死亡、艾滋病或超过50%的CD4细胞计数下降50%,使用艾滋病临床试验组175(对双重治疗)和ACTG320(双重治疗和三重治疗)的数据。我们审查一套识别假设并估计风险差异。我们使用一个反概率加权估计器,利用共同试用武器(双重治疗)进行计算。根据对共用武器进行比较后提出的聚合诊断分析可能表明违反识别假设。数据集算和诊断对ACTG试验的应用表明,与个人单项治疗相比,风险减少的三倍结果是50至300个细胞/立方米之间的单项治疗。我们审查一套识别假设比较方法,解决了每个组成数据源都无法单独处理的问题,但需要仔细考虑。