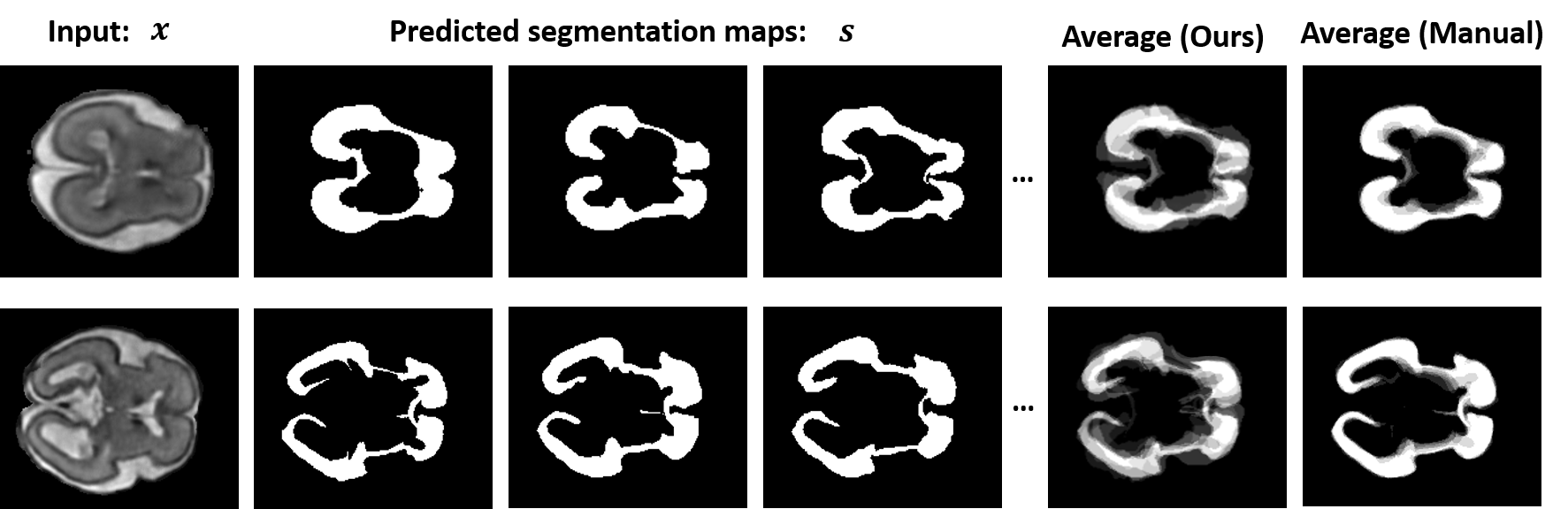
Lesions or organ boundaries visible through medical imaging data are often ambiguous, thus resulting in significant variations in multi-reader delineations, i.e., the source of aleatoric uncertainty. In particular, quantifying the inter-observer variability of manual annotations with Magnetic Resonance (MR) Imaging data plays a crucial role in establishing a reference standard for various diagnosis and treatment tasks. Most segmentation methods, however, simply model a mapping from an image to its single segmentation map and do not take the disagreement of annotators into consideration. In order to account for inter-observer variability, without sacrificing accuracy, we propose a novel variational inference framework to model the distribution of plausible segmentation maps, given a specific MR image, which explicitly represents the multi-reader variability. Specifically, we resort to a latent vector to encode the multi-reader variability and counteract the inherent information loss in the imaging data. Then, we apply a variational autoencoder network and optimize its evidence lower bound (ELBO) to efficiently approximate the distribution of the segmentation map, given an MR image. Experimental results, carried out with the QUBIQ brain growth MRI segmentation datasets with seven annotators, demonstrate the effectiveness of our approach.
翻译:通过医学成像数据可见的遗迹或器官界限往往模糊不清,从而导致多阅读器划界方面的显著差异,即偏差不确定性的来源,特别是量化磁共振成像数据人工说明的观测器间变异性,在为各种诊断和治疗任务建立参考标准方面起着关键作用。不过,多数分解方法只是将图象建模到单一的分解图中,不考虑说明器的分歧。为了在不牺牲准确性的情况下考虑观察器之间的变异性,我们建议了一个新的变异性推断框架,以模型形式显示可信的分解图的分布,以具体的MR图像为模型,明确代表多读器变异性。具体地说,我们利用一种潜在的矢量矢量来编码多读变异性和抵消成像数据中固有的信息损失。然后,我们应用一个变异性自动电解码网络,并优化其较低约束性的证据(ELBO),以高效地接近分解图的分布,以MR图像为基准,我们提出了一个新的变异性推导结果,与7个脑分段方法演示了我们的MQ。