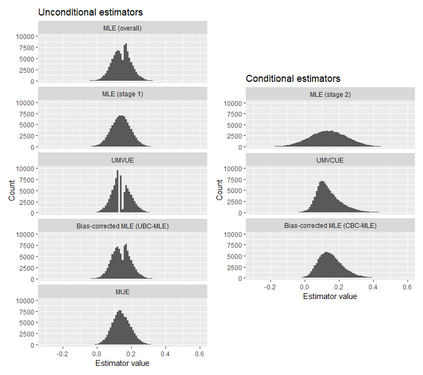
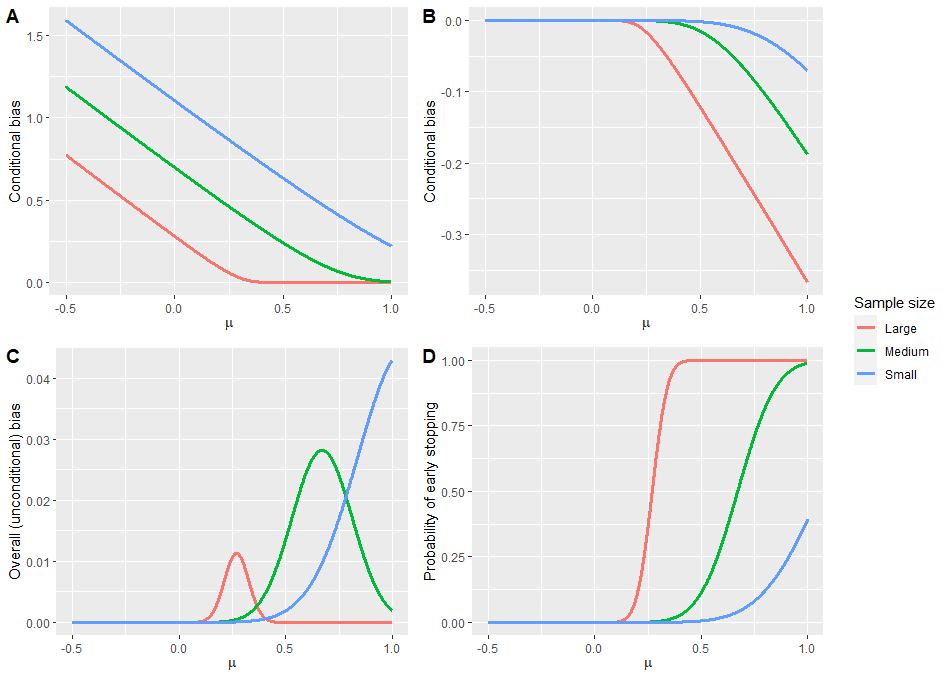
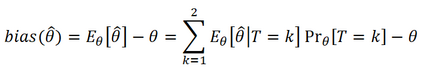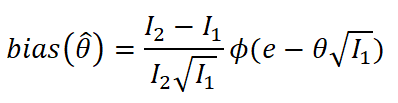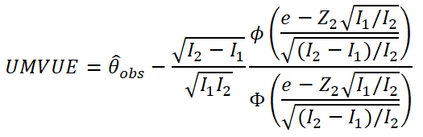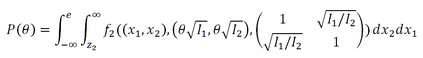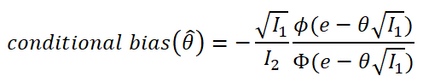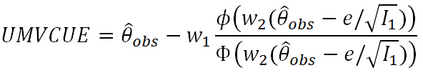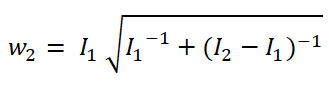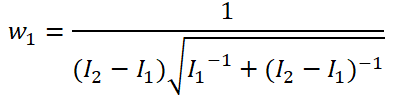In adaptive clinical trials, the conventional end-of-trial point estimate of a treatment effect is prone to bias, that is, a systematic tendency to deviate from its true value. As stated in recent FDA guidance on adaptive designs, it is desirable to report estimates of treatment effects that reduce or remove this bias. However, it may be unclear which of the available estimators are preferable, and their use remains rare in practice. This paper is the second in a two-part series that studies the issue of bias in point estimation for adaptive trials. Part I provided a methodological review of approaches to remove or reduce the potential bias in point estimation for adaptive designs. In part II, we discuss how bias can affect standard estimators and assess the negative impact this can have. We review current practice for reporting point estimates and illustrate the computation of different estimators using a real adaptive trial example (including code), which we use as a basis for a simulation study. We show that while on average the values of these estimators can be similar, for a particular trial realisation they can give noticeably different values for the estimated treatment effect. Finally, we propose guidelines for researchers around the choice of estimators and the reporting of estimates following an adaptive design. The issue of bias should be considered throughout the whole lifecycle of an adaptive design, with the estimation strategy pre-specified in the statistical analysis plan. When available, unbiased or bias-reduced estimates are to be preferred.
翻译:在适应性临床试验中,传统的审终点对治疗效果的估计容易产生偏差,即有偏离其真正价值的系统性趋势。正如林业发展局最近的适应性设计指南所述,最好报告对减少或消除这种偏差的治疗作用的估计,然而,可能不清楚现有估算者中哪一种更可取,实际中仍然很少使用。本文是研究对适应性试验进行点估测的偏差问题的两部分系列中的第二份文件。第一部分对消除或减少对适应性设计进行点估测的潜在偏差的方法进行了方法审查。在第二部分中,我们讨论了偏见如何影响标准估测员并评估其可能产生的负面影响。我们审查报告点估算的现行做法,并用实际的适应性试验实例(包括代码)说明不同估算者的计算方法。我们用这个实例作为模拟研究的基础。我们表明,平均而言,这些估算者的价值可以相似,在特定试验中,它们可以明显地给估计的治疗效果带来不同的价值。最后,我们为研究人员提出了有关标准估测算结果的指南。我们审查了目前的报告点估计点估计点估计值,应该在整个统计周期中选择一种统计性估算方法。我们考虑采用整个评估周期前的估价方法。