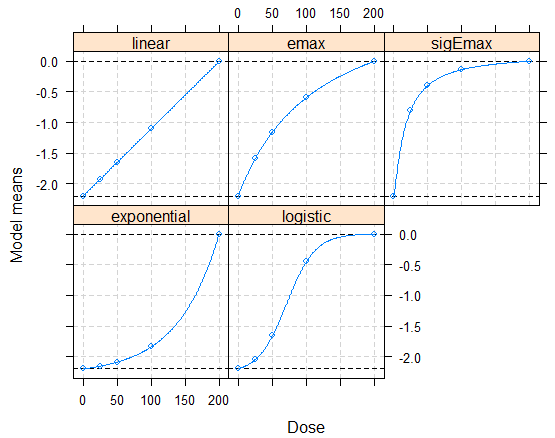
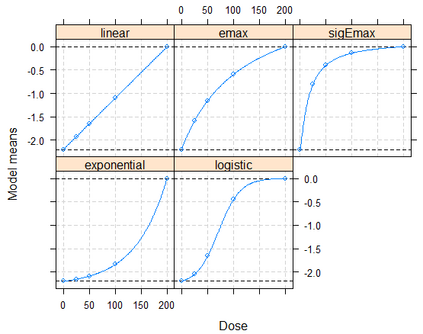
Bretz et al (2005) proposed multiple Comparison Procedure and Modeling (MCPMod) method to design and analyze dose-finding study. Pinheiro (2014) then generalized it to various types of endpoint, including but not limited to binary endpoint, survival endpoint, count data, and longitudinal data. Pinheiro (2013) recommended to use the estimated covariance matrix from the observed data to recalculate the optimal contrast and the critical value of the test For many phase II studies it is common to have small sample sizes per arm with low placebo response rates jointly. Under such circumstances, it cannot be excluded to have a zero count observed. For example, when the placebo response rate is 10%, there is about 4% chance to observe zero responders in the placebo group, or other dose group(s), which has a similar response rate as placebo. In this manuscript, we would like to illustrate the potential problem of Pinheiro (2013) using a case study and simulations. An alternative method using Firth's logistic regression was evaluated to get a stable estimate of response for each dose group. In addition, we evaluated two options to address the issue with problematic contrast coefficients.
翻译:Bretz等人(2005年)提出了多种比较程序和模型(MCPMod)方法,用于设计和分析剂量调查研究。皮涅罗(2014年)随后将其推广到各种类型的终点,包括但不限于二进制终点、生存端点、计数数据和纵向数据。皮涅罗(2013年)建议使用观察到的数据的估计共变矩阵,重新计算最佳对比和试验的关键价值。许多第二阶段的研究都常见于每个手臂的样本大小较小,而且安眠药反应率较低。在这种情况下,不能排除观察到零计数。例如,在安眠药反应率为10%的情况下,观察到安眠药组或其他剂量组的零反应者的机会约为4%,后者的反应率与安眠药相近。在这个手稿中,我们想用案例研究和模拟来说明皮涅罗(2013年)的潜在问题。对使用Firth物流回归法的替代方法进行了评估,以便对每一剂量组的反应作出稳定估计。此外,我们评估了用有问题对比系数处理该问题的两种选择。