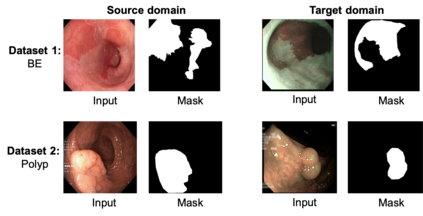
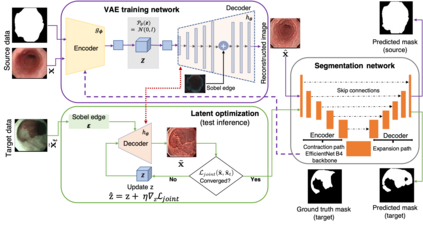
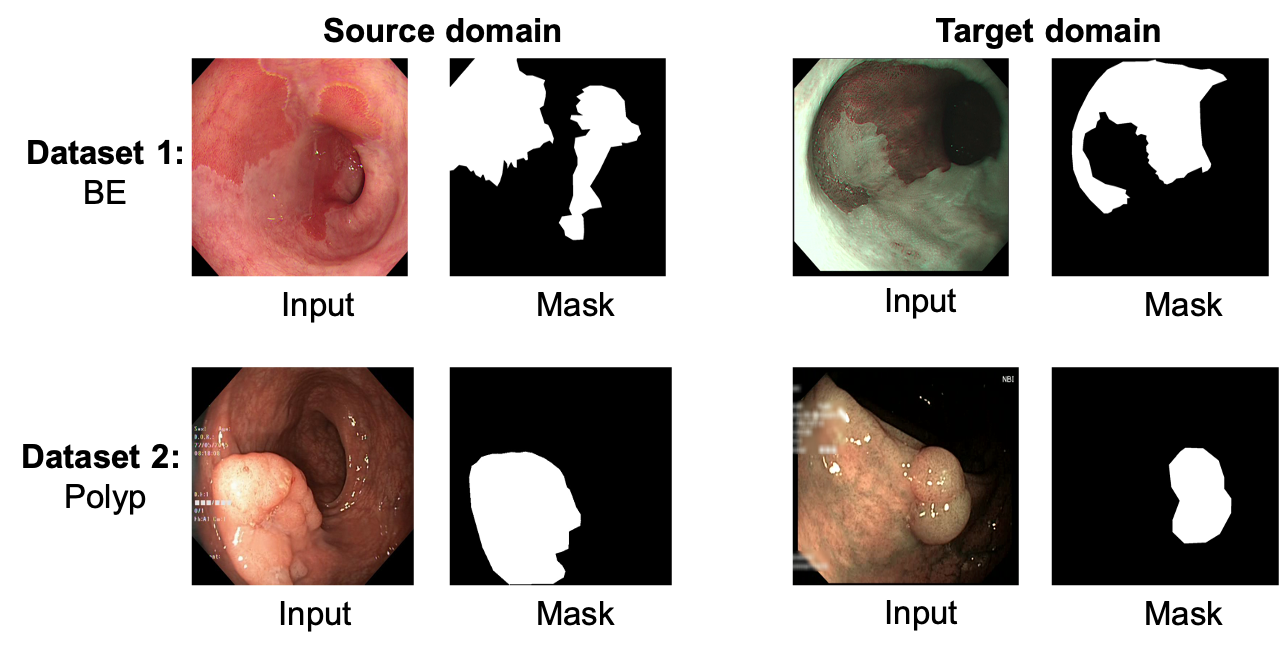
Gastrointestinal (GI) cancer precursors require frequent monitoring for risk stratification of patients. Automated segmentation methods can help to assess risk areas more accurately, and assist in therapeutic procedures or even removal. In clinical practice, addition to the conventional white-light imaging (WLI), complimentary modalities such as narrow-band imaging (NBI) and fluorescence imaging are used. While, today most segmentation approaches are supervised and only concentrated on a single modality dataset, this work exploits to use a target-independent unsupervised domain adaptation (UDA) technique that is capable to generalize to an unseen target modality. In this context, we propose a novel UDA-based segmentation method that couples the variational autoencoder and U-Net with a common EfficientNet-B4 backbone, and uses a joint loss for latent-space optimization for target samples. We show that our model can generalize to unseen target NBI (target) modality when trained using only WLI (source) modality. Our experiments on both upper and lower GI endoscopy data show the effectiveness of our approach compared to naive supervised approach and state-of-the-art UDA segmentation methods.
翻译:在临床实践中,除了传统的白光成像(WLI)外,还使用了诸如窄带成像(NBI)和荧光成像(荧光成像)等辅助模式。虽然今天大多数分解方法都受到监督,而且只集中在单一模式数据集上,但这项工作利用了一种目标独立的、不受监督的域适应技术,能够概括到一种看不见的目标模式。在这方面,我们提议一种基于UDA的新型分解方法,将变异自动编码器和具有通用高效Net-B4主干线的U-Net结合起来,并使用共同损失来优化目标样本的潜层空间。我们表明,我们的模型在仅使用WLI(源)模式进行培训时,可以概括到不可见的NBI(目标)模式。我们关于GI上下底镜学数据的实验表明我们的方法相对于天性监督方法和状态UDA部分分析方法的有效性。