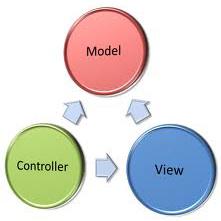
Proper experimental record-keeping is an important cornerstone in research and development for the purpose of auditing. The gold standard of record-keeping is based on the judicious use of physical, permanent notebooks. However, advances in technology had resulted in large amounts of electronic records making it virtually impossible to maintain a full set of records in physical notebooks. Electronic laboratory notebook systems aim to meet the stringency for keeping records electronically. This manuscript describes CyNote which is an electronic laboratory notebook system that is compliant with 21 CFP Part 11 controls on electronic records, requirements set by USA Food and Drug Administration for electronic records. CyNote is implemented on web2py framework and is adhering to the architectural paradigm of model-view-controller (MVC), allowing for extension modules to be built for CyNote. CyNote is available at http://cynote.sf.net.
翻译:适当的实验记录保存是审计目的研发工作的一个重要基石,记录保存金标准的基础是明智地使用实物、永久笔记本,然而,技术的进步导致大量电子记录,使得几乎不可能在实物笔记本中保存全套记录。电子实验室笔记本系统旨在满足电子记录保存的严格性。这份手稿描述了CyNot,这是一个电子实验室笔记本系统,符合21个CFP第11部分对电子记录的控制,美国食品和药品管理局对电子记录的要求。CyNot在Web2py框架内实施,并遵守了示范视控器(MVC)的建筑范式,允许为CyNot建立扩展模块。CyNot可在http://cynote.sf.net上查阅。