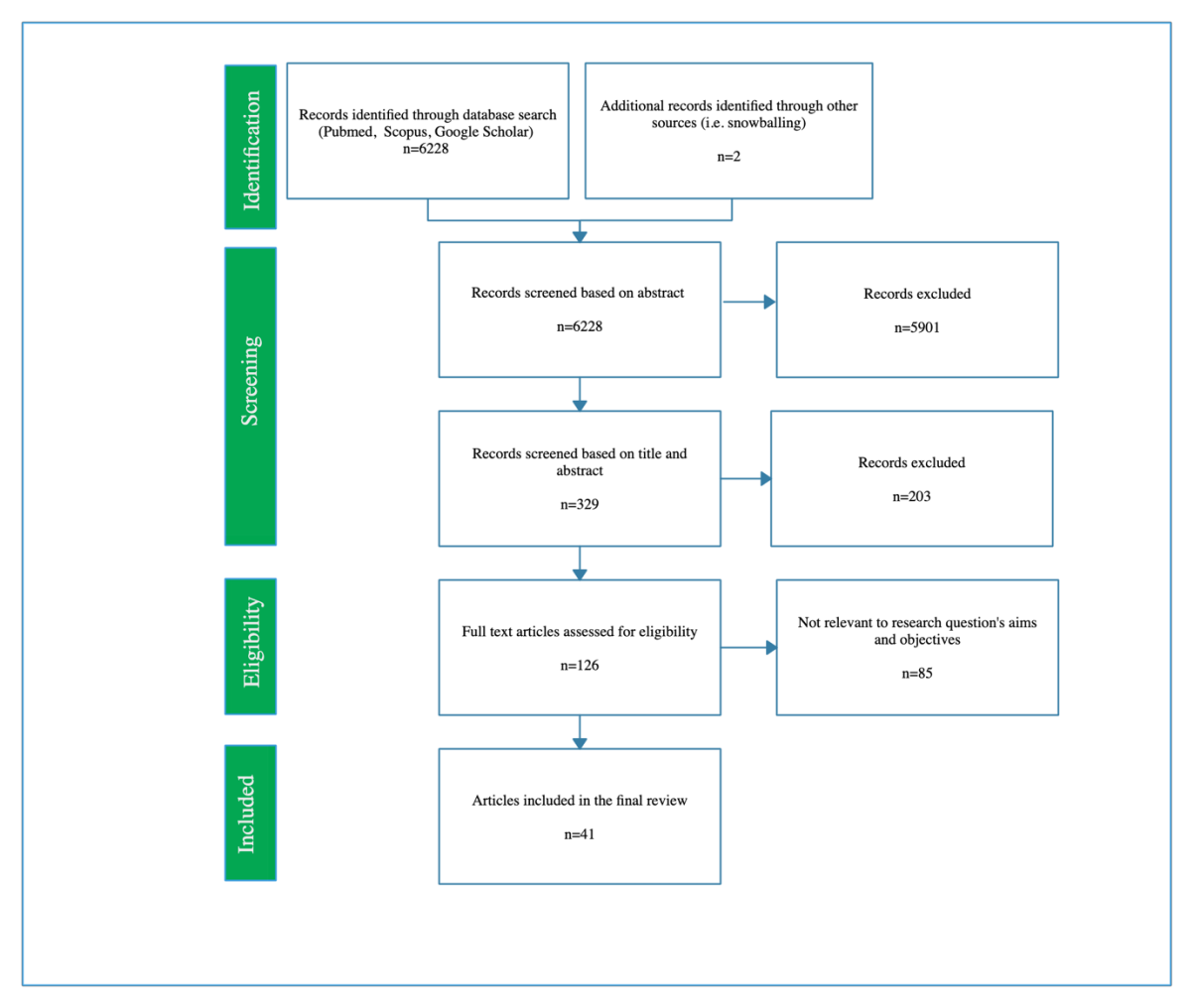
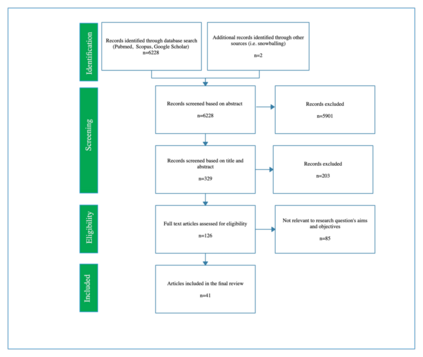
The potential presented by Artificial Intelligence (AI) for healthcare has long been recognised by the technical community. More recently, this potential has been recognised by policymakers, resulting in considerable public and private investment in the development of AI for healthcare across the globe. Despite this, excepting limited success stories, real-world implementation of AI systems into front-line healthcare has been limited. There are numerous reasons for this, but a main contributory factor is the lack of internationally accepted, or formalised, regulatory standards to assess AI safety and impact and effectiveness. This is a well-recognised problem with numerous ongoing research and policy projects to overcome it. Our intention here is to contribute to this problem-solving effort by seeking to set out a minimally viable framework for evaluating the safety, acceptability and efficacy of AI systems for healthcare. We do this by conducting a systematic search across Scopus, PubMed and Google Scholar to identify all the relevant literature published between January 1970 and November 2020 related to the evaluation of: output performance; efficacy; and real-world use of AI systems, and synthesising the key themes according to the stages of evaluation: pre-clinical (theoretical phase); exploratory phase; definitive phase; and post-market surveillance phase (monitoring). The result is a framework to guide AI system developers, policymakers, and regulators through a sufficient evaluation of an AI system designed for use in healthcare.
翻译:长期以来,技术界一直认识到人工智能(AI)对卫生保健的潜力,最近,决策者认识到了这种潜力,因此在全球范围对开发人工智能保健进行大量公共和私人投资。尽管如此,除了有限的成功事例外,实际将人工智能系统应用于一线卫生保健有限,但实际实施人工智能系统有限,原因很多,但主要促成因素之一是缺乏国际公认的或正式的监管标准来评估人工智能的安全、影响和有效性。这是一个得到广泛承认的问题,许多正在进行的研究和政策项目都正在解决这一问题。我们的意图是,通过努力为评估保健方面人工智能系统的安全、可接受性和有效性制定一个最起码可行的框架,为解决问题作出贡献。我们这样做的方式是在斯科普斯、普布迈德和谷歌学者之间进行系统搜索,以确定1970年1月至2020年11月期间公布的与评估:产出绩效、功效、实际使用人工智能系统有关的所有相关文献。这是一个问题,根据评估阶段,即临床前、后期和后期的监测系统,综合了关键主题。一个探索阶段是,一个完整的国际监测系统,一个完整的分析阶段;一个完整的分析阶段,一个完整的分析阶段,一个进入国际信息系统;一个完整的分析阶段;一个完整的分析阶段;一个完整的分析阶段;一个完整的分析阶段,一个分析阶段;一个完整的分析阶段,一个完整的分析阶段,一个分析阶段,一个完整的分析阶段;一个完整的分析阶段;一个分析阶段;一个完整的分析阶段;一个完整的分析阶段;一个完整的分析阶段;一个完整的分析阶段;一个分析阶段。