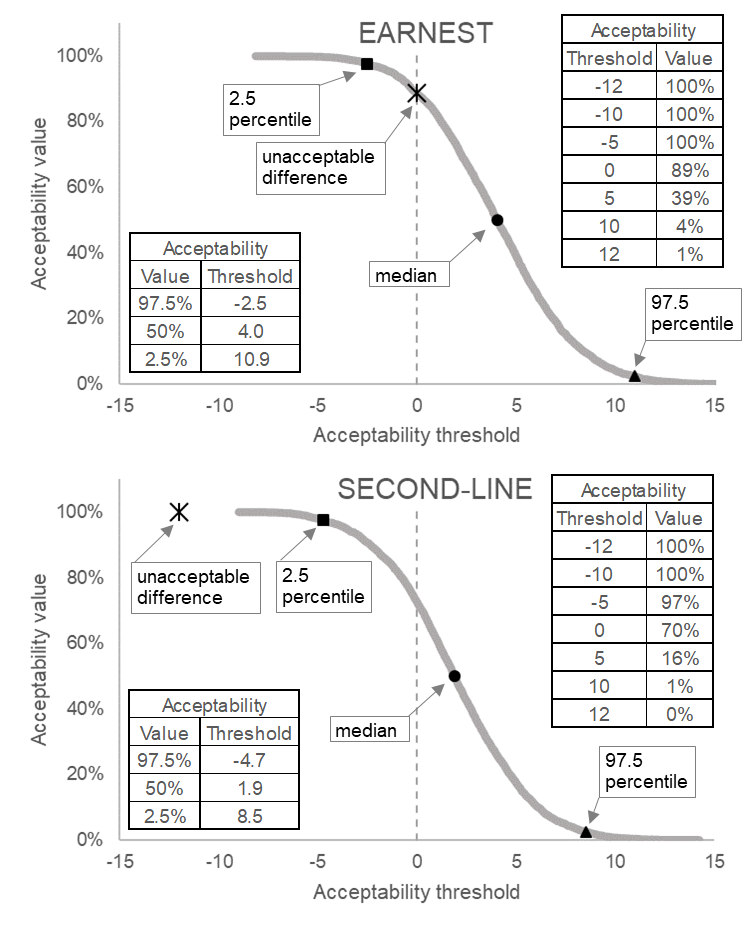
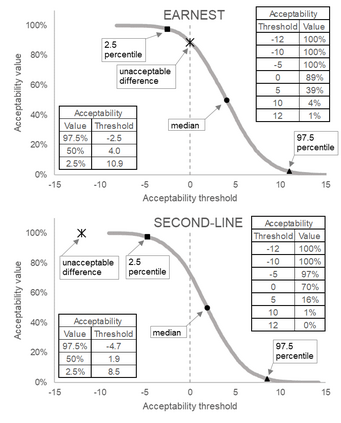
Effective decision making from randomised controlled clinical trials relies on robust interpretation of the numerical results. However, the language we use to describe clinical trials can cause confusion both in trial design and in comparing results across trials. ACceptability Curve Estimation using Probability Above Threshold (ACCEPT) aids comparison between trials (even where of different designs) by harmonising reporting of results, acknowledging different interpretations of the results may be valid in different situations, and moving the focus from comparison to a pre-specified value to interpretation of the trial data. ACCEPT can be applied to historical trials or incorporated into statistical analysis plans for future analyses. An online tool enables ACCEPT on up to three trials simultaneously.
翻译:随机控制的临床试验的有效决策取决于对数字结果的可靠解释,然而,我们用来描述临床试验的语言在审判设计和比较各审判结果时都可能造成混乱。 利用概率高于阈值的概率(ACCEPT)进行感知曲线估计有助于对试验进行比较(即使在不同设计的情况下),方法是统一报告结果,承认对结果的不同解释在不同情况下可能有效,并将重点从比较转向对试验数据的预定价值。 ACCEPT可以适用于历史试验,也可以纳入统计分析计划,以便今后进行分析。 在线工具使ACEPT能够同时进行多达三种试验。