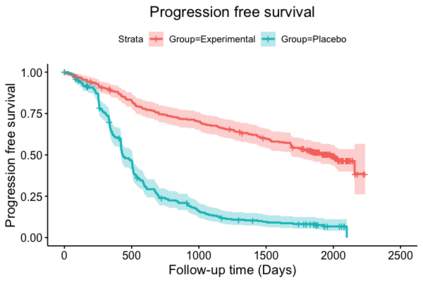
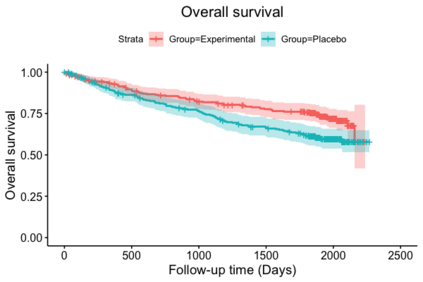
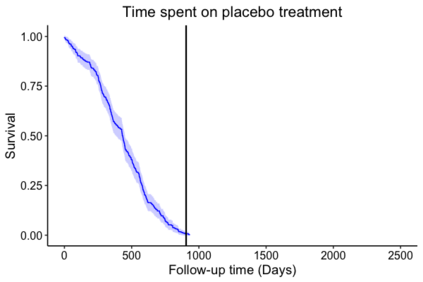
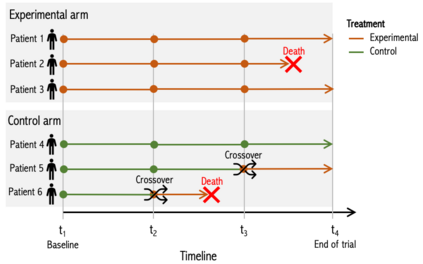
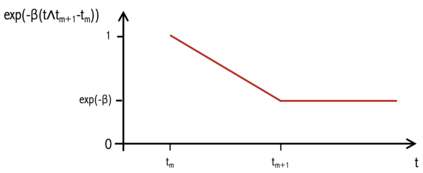
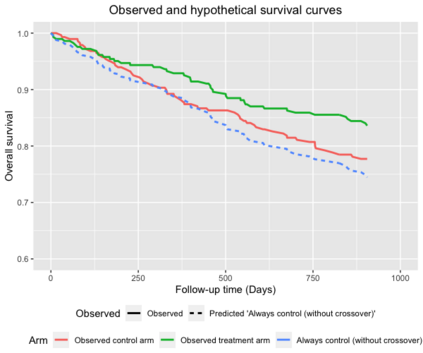
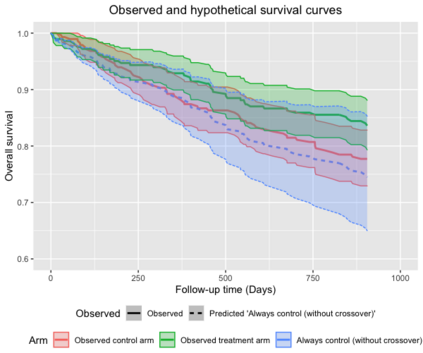
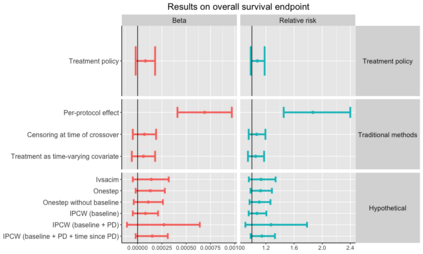
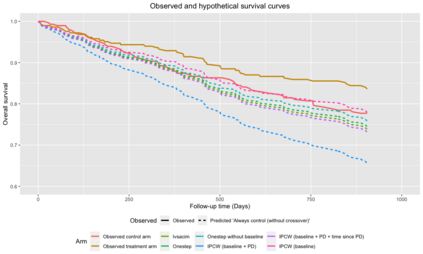
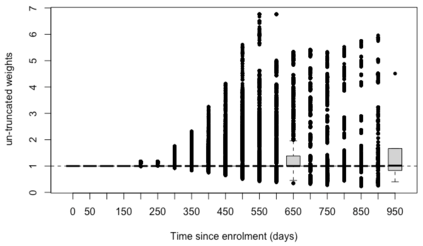
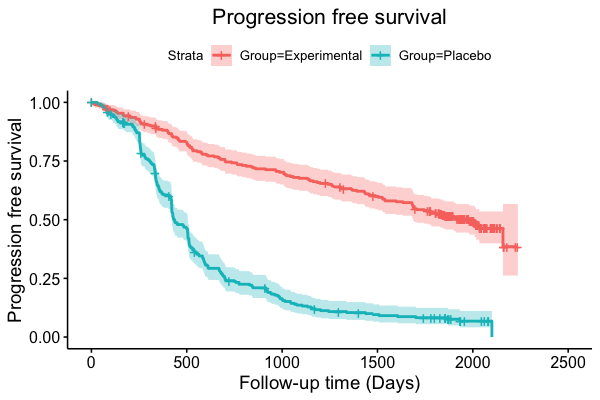
When choosing estimands and estimators in randomized clinical trials, caution is warranted as intercurrent events, such as - due to patients who switch treatment after disease progression, are often extreme. Statistical analyses may then easily lure one into making large implicit extrapolations, which often go unnoticed. We will illustrate this problem of implicit extrapolations using a real oncology case study, with a right-censored time-to-event endpoint, in which patients can cross over from the control to the experimental treatment after disease progression, for ethical reasons. We resolve this by developing an estimator for the survival risk ratio contrasting the survival probabilities at each time t if all patients would take experimental treatment with the survival probabilities at those times t if all patients would take control treatment up to time t, using randomization as an instrumental variable to avoid reliance on no unmeasured confounders assumptions. This doubly robust estimator can handle time-varying treatment switches and right-censored survival times. Insight into the rationale behind the estimator is provided and the approach is demonstrated by re-analyzing the oncology trial.
翻译:在随机临床试验中选择估计值和估计值时,必须谨慎行事,因为间歇性事件,例如,由于病人在病变后转诊治疗而导致的病人,往往是极端的; 统计分析可能很容易地诱使一个人进行大规模的隐含外推,而这种外推往往不为人注意。 我们将利用真正的肿瘤学案例研究来说明隐含的外推问题,同时使用一种正确的检查时间到时间的假设,使病人能够从控制到疾病不断演变后的实验治疗,这是合乎道德的。我们为此开发一个生存风险比估计器,对比每一次存活概率,如果所有病人在当时都以生存概率来进行实验治疗,如果所有病人都能控制治疗到时间,则使用随机化作为工具变量,避免依赖任何未经测量的断裂假设。这个加倍有力的估计器可以处理时间变化治疗开关和正确检测的存活时间。如果对所有病人进行观察,那么对测算仪背后的原理进行推算,并且通过重新测试来证明方法。</s>