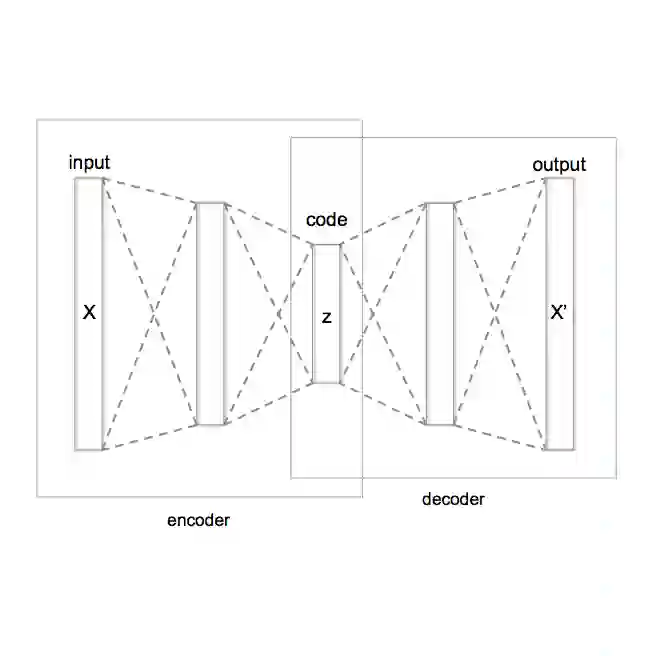
Deep generative chemistry models emerge as powerful tools to expedite drug discovery. However, the immense size and complexity of the structural space of all possible drug-like molecules pose significant obstacles, which could be overcome with hybrid architectures combining quantum computers with deep classical networks. We built a compact discrete variational autoencoder (DVAE) with a Restricted Boltzmann Machine (RBM) of reduced size in its latent layer. The size of the proposed model was small enough to fit on a state-of-the-art D-Wave quantum annealer and allowed training on a subset of the ChEMBL dataset of biologically active compounds. Finally, we generated $4290$ novel chemical structures with medicinal chemistry and synthetic accessibility properties in the ranges typical for molecules from ChEMBL. The experimental results point towards the feasibility of using already existing quantum annealing devices for drug discovery problems, which opens the way to building quantum generative models for practically relevant applications.
翻译:然而,所有可能的药物类分子结构空间的巨大规模和复杂性构成了巨大的障碍,可以通过混合结构将量子计算机与深层古典网络相结合来克服这些障碍。我们建造了一个紧凑的离散变异自动编码器(DVAE),其潜层尺寸受限的Boltzmann机器(RBM),其规模小到足以适应最先进的D-Wave量子annealer的尺寸,并允许就CEMBL生物活性化合物数据集进行一组培训。最后,我们生成了4290亿美元的新型化学结构,这些化学结构具有医药化学特性,合成可进入CEEMBL分子的典型范围。实验结果表明,使用现有量子射精装置解决药物发现问题的可行性,这为建立与实际相关的量子化模型开辟了道路。