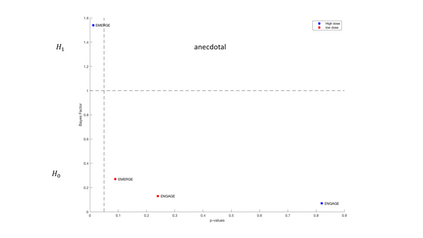
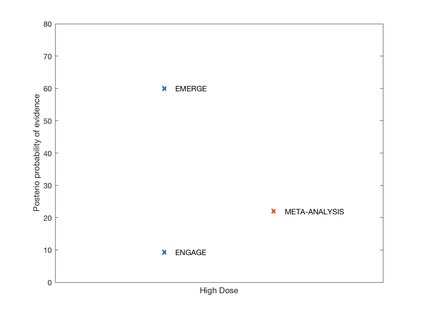
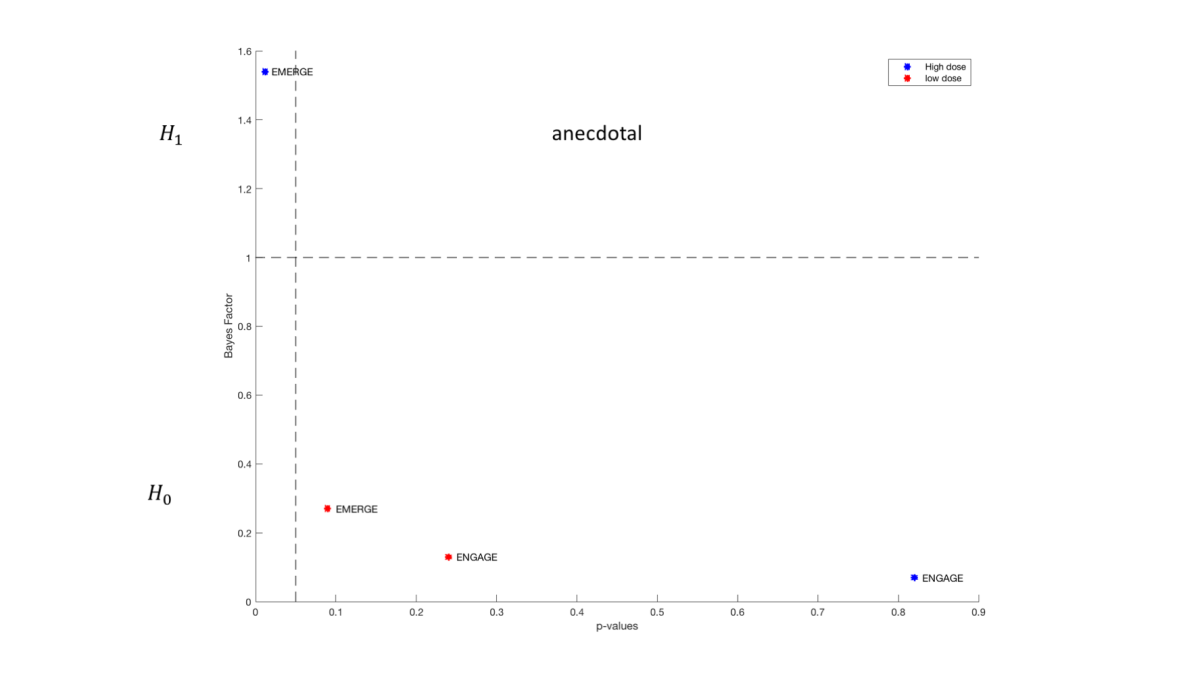
In this article we have conducted a reanalysis of the phase III aducanumab (ADU) summary statistics announced by Biogen, in particular the result of the Clinical Dementia Rating-Sum of Boxes (CDR-SB). The results showed that the evidence on the efficacy of the drug is very low and a more clearer view of the results of clinical trials are presented in the Bayesian framework that can be useful for future development and research in the field.
翻译:在本篇文章中,我们重新分析了生物基因宣布的第三阶段Aducanumab(ADU)简要统计数据,特别是临床痴呆症评级-箱状细胞(CDR-SB)的结果,结果表明,关于药物功效的证据非常低,巴伊西亚框架更清楚地介绍了临床试验结果,这对这一领域的未来发展和研究可能有用。
相关内容
专知会员服务
28+阅读 · 2020年2月18日
专知会员服务
11+阅读 · 2020年1月17日
专知会员服务
36+阅读 · 2019年10月17日
Arxiv
0+阅读 · 2021年9月8日
Arxiv
4+阅读 · 2021年3月29日
Arxiv
15+阅读 · 2020年12月3日
Arxiv
3+阅读 · 2020年7月3日