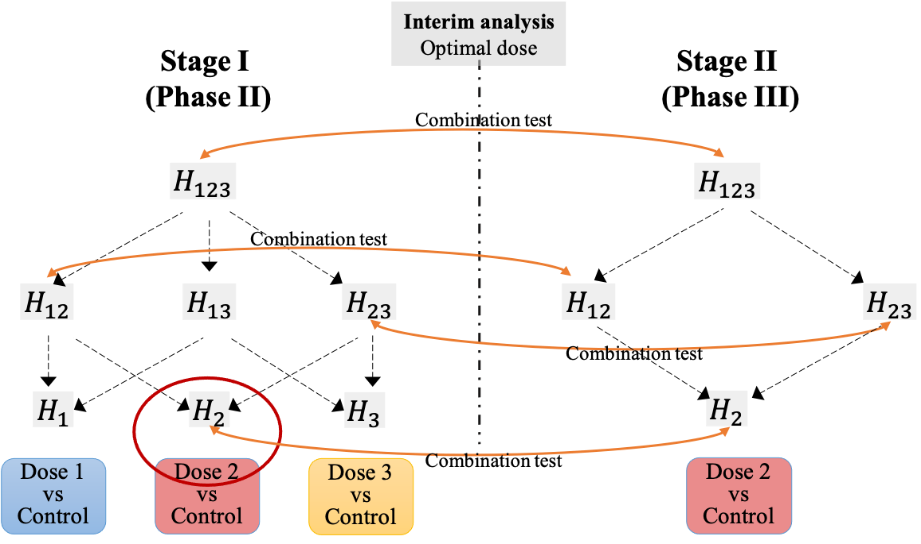
The traditional more-is-better dose selection paradigm, developed based on cytotoxic chemotherapeutics, is often problematic When applied to the development of novel molecularly targeted agents (e.g., kinase inhibitors, monoclonal antibodies, and antibody-drug conjugates). The US Food and Drug Administration (FDA) initiated Project Optimus to reform the dose optimization and dose selection paradigm in oncology drug development and call for more attention to benefit-risk consideration. We systematically investigated the operating characteristics of the seamless phase 2-3 design as a strategy for dose optimization, where in stage 1 (corresponding to phase 2) patients are randomized to multiple doses, with or without a control; and in stage 2 (corresponding to phase 3) the efficacy of the selected optimal dose is evaluated with a randomized concurrent control or historical control. Depending on whether the concurrent control is included and the type of endpoints used in stages 1 and 2, we describe four types of seamless phase 2-3 dose-optimization designs, which are suitable for different clinical settings. The statistical and design considerations that pertain to dose optimization are discussed. Simulation shows that dose optimization phase 2-3 designs are able to control the familywise type I error rates and yield appropriate statistical power with substantially smaller sample size than the conventional approach. The sample size savings range from 16.6% to 27.3%, depending on the design and scenario, with a mean savings of 22.1%. Due to the interim dose selection, the phase 2-3 dose-optimization design is logistically and operationally more challenging, and should be carefully planned and implemented to ensure trial integrity.
翻译:美国食品和药品管理局(FDA)启动了基于细胞毒性助产疗效的更传统的更好剂量选择模式。 当应用到开发新的分子定向物剂(如:脂酶抑制剂、单克隆抗体和抗体药物合金)时,通常会成问题。美国食品和药品管理局(FDA)启动了项目Optimus,以改革肿瘤药物开发中的剂量优化和剂量选择模式,并呼吁更多地关注利益风险考虑。我们系统调查了无缝2-3级设计的运作特点,作为剂量优化战略,在第一阶段(对2级过渡性反应),病人随机按多种剂量(有或无控制);在第二阶段(对抗体抗体抗体抗体抗体抗体抗体抗体抗体),对选定最佳剂量的功效进行评估,取决于是否包括同时的管制以及第1阶段和第2阶段使用的终点类型。我们描述了四类无缝的2-3级度抗药效度强化剂量设计,适合不同临床环境。 6级(对2级过渡阶段)病人进行随机随机随机随机随机随机随机随机随机随机随机随机随机随机随机随机随机随机随机随机进行多次评估,, 将精测测测测测测测算,将22级的机精准机精准机精测测测测测测测测测测测测测测测测测测测测测测测测测测测测度为Sir机机机机机型机型机型机型机型机型机型机型机型机型机型,将机型的机型的机型机型机型机型号,将机算机算机算机型精测算至2-3。