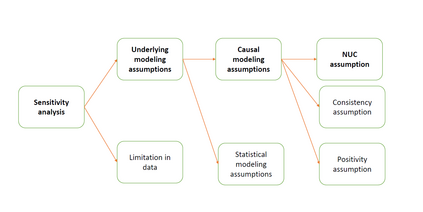
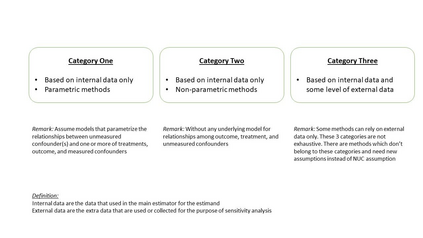
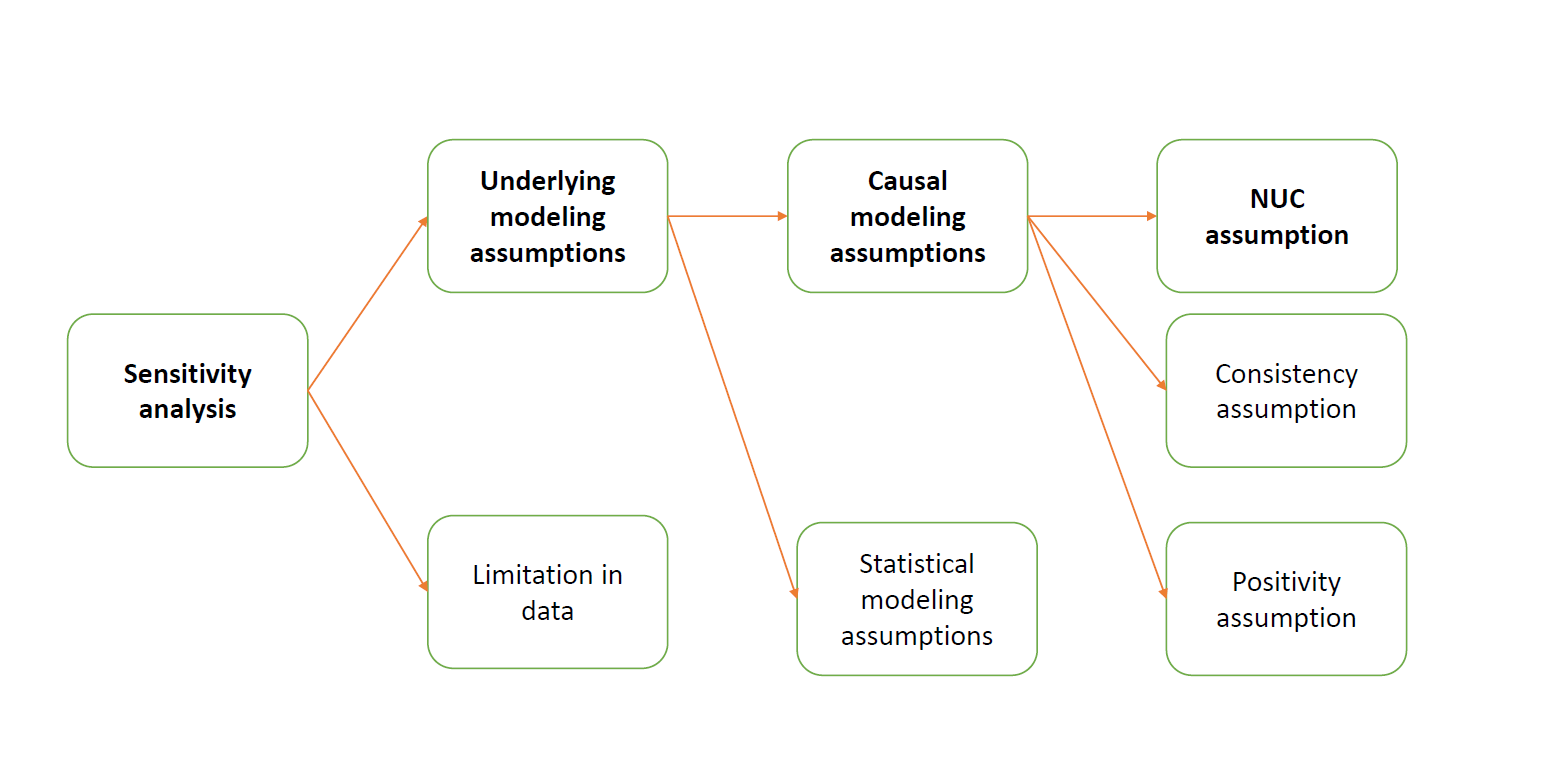
The American Statistical Association Biopharmaceutical Section (ASA BIOP) working group on real-world evidence (RWE) has been making continuous, extended effort towards a goal of supporting and advancing regulatory science with respect to non-interventional, clinical studies intended to use real-world data for evidence generation for the purpose of medical product development and evaluation (i.e., RWE studies). In 2023, the working group published a manuscript delineating challenges and opportunities in constructing estimands for RWE studies following a framework in ICH E9(R1) guidance on estimand and sensitivity analysis. As a follow-up task, we describe the other issue in RWE studies, sensitivity analysis. Focusing on the issue of unmeasured confounding, we review availability and applicability of sensitivity analysis methods for different types unmeasured confounding. We discuss consideration on the choice and use of sensitivity analysis for RWE studies. Updated version of this article will present how findings from sensitivity analysis could support regulatory decision-making using a real example.
翻译:暂无翻译