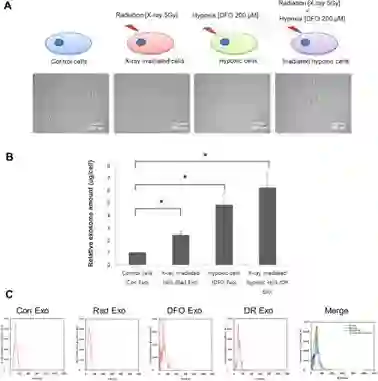
磁性粒子成像系统追踪外泌体在缺氧肿瘤组织中的摄取
肿瘤组织的缺氧区域治疗仍然是一个重大挑战。近日, Biomaterials杂志的一项研究中,来自斯坦福大学的研究人员开发一种能够靶向肿瘤缺氧区域的外泌体平台,并且可以使用磁性粒子成像(MPI)在体内进行监测。从MDA-MB-231人乳腺癌细胞中分离出四种类型的外泌体(细胞在低氧或含氧量正常的条件下,并且分别暴露或不暴露于X射线辐射,这四种调节下产生的外泌体)。用DiO(一种荧光亲脂性示踪剂)标记外泌体,以量化它们被缺氧肿瘤细胞的摄取情况。随后,将外泌体进行修饰使其携带SPIO(超顺磁氧化铁)纳米颗粒和Olaparib(PARP抑制剂)。FACS和荧光显微镜结果显示,与其他外泌体制剂相比,缺氧细胞优先吸收由缺氧细胞释放的外泌体。此外,使用MPI在体内连续成像SPIO标记的外泌体的分布。最后,通过增加的细胞凋亡和体内较慢的肿瘤生长证实了载有Olaparib的外泌体的治疗功效。这种新颖的诊断平台可以作为一种有效的策略来监测外泌体并将治疗药物递送至缺氧肿瘤组织。
图:四种处理细胞分泌的外泌体的定量定性分析
文章摘要:
Treating the hypoxic region of the tumorremains a significant challenge. The goals of this study are to develop anexosome platform that can target regions of tumor hypoxia and that can bemonitored in vivo using magnetic particle imaging (MPI). Four types of exosomes(generated under hypoxic or normoxic conditions, and with or without exposureto X-ray radiation) were isolated from MDA-MB-231 human breast cancer cells.Exosomes were labeled by DiO, a fluorescent lipophilic tracer, to quantifytheir uptake by hypoxic cancer cells. Subsequently, the exosomes were modifiedto carry SPIO (superparamagnetic iron oxide) nanoparticles and Olaparib (PARPinhibitor). FACS and fluorescence microscopy showed that hypoxic cellspreferentially take up exosomes released by hypoxic cells, compared with otherexosome formulations. In addition, the distribution of SPIO-labeled exosomeswas successively imaged in vivo using MPI. Finally, the therapeutic efficacy ofOlaparib-loaded exosomes was demonstrated by increased apoptosis and slowertumor growth in vivo. Our novel theranostic platform could be used as aneffective strategy to monitor exosomes in vivo and deliver therapeutics tohypoxic tumors.
参考文献:
Jung KO, Jo H, Yu JH, Gambhir SS, Pratx G. (2018) Development andMPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials177:139-148.
科研学习班了解一下~ ~(点击详细了解):
MATLAB高级编程及机器学习技术应用研讨班
回复“外泌体” 阅读外泌体最新科研进展及动态
回复“EV” 阅读 2016-2018年This Week in Extracellular Vesicles
回复“盘点” 阅读 外泌体领域十大前沿进展盘点