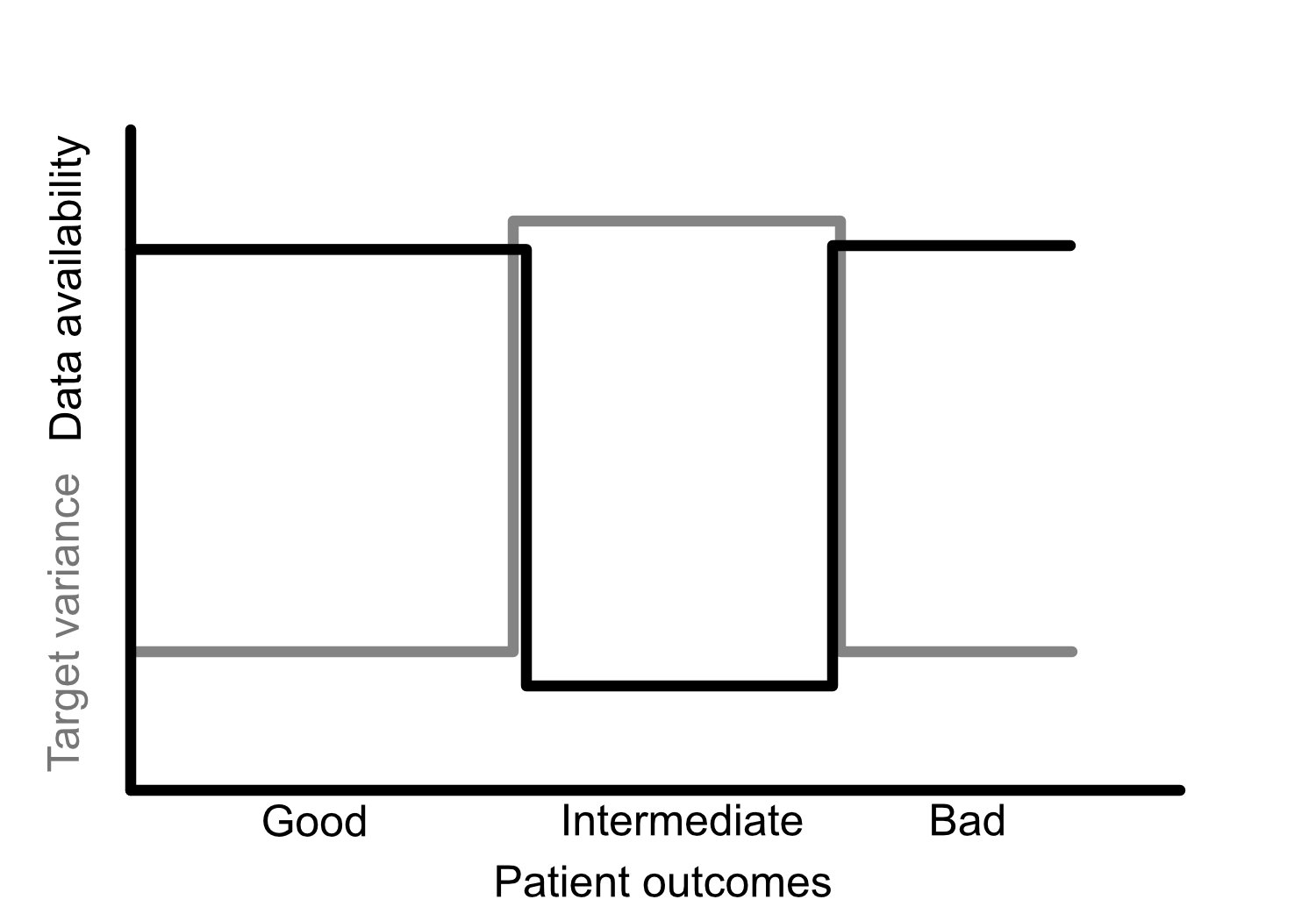
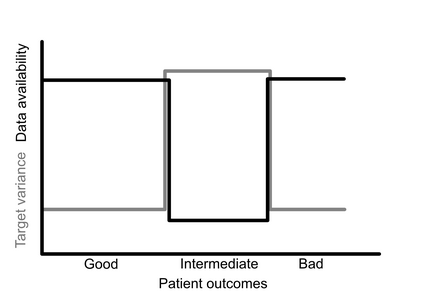
Medical AI products require certification before deployment in most jurisdictions. To date, clear pathways for regulating medical AI are still under development. I present a methodological guide to the development of a regulatory package which will form part of a certification process. This approach is predicated on the translation between a statistical risk perspective, typical of medical device regulators, and a deep understanding of machine learning methodologies. This work of translation envisages the statistician as the key negotiator between medical device regulators and machine learning experts, allowing them to communicate more clearly, and thus lead to the development of standardised pathways for medical AI regulation.
翻译:在大多数司法管辖区,医疗自译自审产品在部署前需要认证。迄今为止,规范医疗自审的明确途径仍在开发中。我为制定监管一揽子方案提供了方法指南,这将构成认证进程的一部分。这种方法的前提是从统计风险角度、医疗装置监管者的典型特点和对机器学习方法的深入理解之间翻译。翻译工作设想统计员作为医疗装置监管者和机器学习专家之间的主要谈判人,使他们能够进行更明确的沟通,从而导致制定医疗自审监管标准化途径。