北大饶毅教授回应:不存在学术造假行为!

近日有人匿名向方舟子举报称“北京大学理学部主任饶毅是中国建国以来隐藏最深的、罪的学术造假骗子”。并列举了其参与的9篇论文的图片造假详细问题,此事在微信群、朋友圈等流传甚广。
方舟子发文
方舟子发文称:饶毅被举报的9篇论文中,有4篇论文他无法判断是否有问题,但是另外5篇论文的确都存在图片复制、粘贴的问题。其中1篇不存在造假,有4篇存在不同程度的造假。不同的实验出现相同的图片数据,有时是制图时的疏忽,不一定是有意的造假。但这4篇论文中涉嫌造假的图片,有的并不是简单的复制、粘贴,而是做了水平翻转的加工,是为了掩盖造假,不可能是疏忽所致,所以是有意的造假。
方舟子列举的部分例子
方舟子表示这些造假论文都是饶毅实验室与管坤良实验室合作的,是哪个实验室、哪个研究人员的责任,外人不得而知,应由内部做进一步的调查。饶毅、管坤良作为实验室负责人,不太可能亲自做实验,所以造假应是学生或博士后所为,但作为实验室负责人应承担监管责任,对造假的论文应该撤回、修改。即使这些论文的实验结果被后来的实验证实,也改变不了造假的事实。
昨日,饶毅教授发表声明,否认对其学术造假的指控。以下为饶毅的个人声明:
有人匿名散发指饶毅参与署名的9篇论文中图片有造假问题。这些文章和图片十几年前来自美国多个实验室的合作,当年参与实验的十几个人现分布于美洲、欧洲、亚洲的多个国家。其中有饶毅作为通讯作者的文章和管坤良作为通讯作者的文章。匿名殃及管坤良实验室发表的论文,其舞剑之意不在管坤良实验室,在此不予理睬。经过细读文章和查询后,在此提供对饶毅需要负质量和监管责任的图片进行的分析。
与实验生物学其他绝大多数教授一样,在完成研究生和博士后训练而领导实验室后,我们两人已多年不做实验,做实验者提供实验结果。但我们作为实验室的领导和论文的作者,对论文内容的真实性,负有监管的责任。我们应该公平、公正对待实验室以前和现在的研究人员,既应该严格要求,也不能无端指责。
我们分析后很清楚:读文章时图和文字一道读就知道,饶毅实验室十几年前研究人员提供(或有提供可能)的图无一有造假问题而需向学术刊物提出撤稿,也无问题需向做实验、提供图片的原实验室研究人员现在的工作单位致信,也不用通知学术同行这些论文有任何误导。饶毅负监管责任的图出现的同样实验同样对照两次呈现的问题,结果真实无造假,属制图不注意,已与《神经科学杂志》商定发表删除其中一处、合并小图(panel)的更正说明。另外,我们这些研究结果都已经得到很多实验室的验证。
国际规范及其先例
实验室负责人处理实验室造假问题,有国际常规。为简便起见在此以一个比较熟知的先例说明国际规范。
现任美国最大的生物医学研究机构首脑(美国国立健康研究院NIH院长)为Francis Collins(以下简称柯林斯)博士。
1996年,柯林斯任人类基因组研究中心时,发现其实验室一位研究生在1995和1996年以造假的数据写了两篇论文、发表在《美国科学院院刊》(简称PNAS),其第一作者为Amitov Hajra(密西根大学的研究生,在柯林斯实验室进行研究工作),通讯作者为柯林斯本人。柯林斯发现第一作者蓄意大规模造假,因此去信PNAS的杂志提出撤销文章。《芝加哥论坛》(Chicago Tribune)和《纽约时报》都对此有报道。
柯林斯认为自己再仔细也不太可能阻止Hajra的造假(《纽约时报》1996年10月30日报道其原话为:“My bottom-line answer is very unsatisfying, but there is no fail-safe way to prevent this kind of occurrence if a capable, bright, motivated trainee is determined to fabricate data in a deceptive and intentional way, short of setting up a police state in your laboratory,” Dr. Collins said.),但柯林斯事后承担对实验室质量监管的责任。
柯林斯于1993年至1997年任NIH下属的人类基因组中心主任。1997年人类基因组研究中心改为人类基因组研究所,柯林斯任其所长至2008年。2009年,柯林斯被欧巴马总统提升为NIH的院长。
文章列表
饶毅当时在美国圣路易斯的华盛顿大学(和后来美国芝加哥的西北大学)、管坤良当时在美国密西根大学,我们有合作。
只有一篇文章(论文4)有北京大学,因作为三位通讯作者之一的饶毅那时回国了,但做实验的场所和其他作者(包括第一、第二作者和其他两位通讯作者)全部都在美国。
列表如下,此后将分别简称为论文1、论文2、论文3、论文4、论文5。
1) Liu G, Li W, Gao X, Li X, Jurgensen C, Park HT, Shin NY, Yu J, He ML, Hanks SK, Wu JY, Guan KL, and Rao Y. (2007). p130(CAS) is required for netrin signaling and commissural axon guidance. J Neurosci 27:957-68.
2)Liu G, Beggs H, Jürgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu JY, and Rao Y (2004). Netrin requires the focal adhesion kinase and the Src family kinases to induce axon outgrowth and to attract axons. Nature Neurosci 7:1222-1232.
3) Li W, Aurandt J, Jürgensen C, Rao Y, and Guan KL (2006). FAK and Src kinases are required for netrin-induced tyrosine phosphorylation of UNC5. J Cell Sci 119:47-55.
4)Liu G, Li W, Wang L, Kar A, Guan KL, Rao Y, and Wu JY. (2009). DSCAM functions as a netrin receptor in commissural axon pathfinding. Proc Natl Acad Sci USA 106:2951-6.
5) Li W, Lee J, Vikis HG, Lee S-H, Liu G., Aurandt J, Shen T-L, Fearon ER, Guan J-L, Han M, Rao Y, Hong K, and Guan KL (2004). Activation of FAK and Src are receptor proximal events required for netrin signaling. Nature Neurosci 7:1213-1221.
分析和调查
原指控的第6、7、8、9等四篇文章,不存在需要解释的问题。论文1、2、4的第一作者都是Guofa Liu。但他在14年前是做神经导向实验,而不做生物化学,所以讨论的图片不会来自于他。
这些文章是十几年前的,与其调查14年前参与过这篇论文的所有研究人员(现在分布在美国、德国、朝鲜等国的至少10个城市),不如先仔细看看有没有问题,有什么问题,再找他们。而分析后可以看到:完全没有资料真实性问题,也就是完全没有造假问题,所以不用找这些人。
第1、2、3、4、5篇等五篇文章,图片问题分析的结果如下:
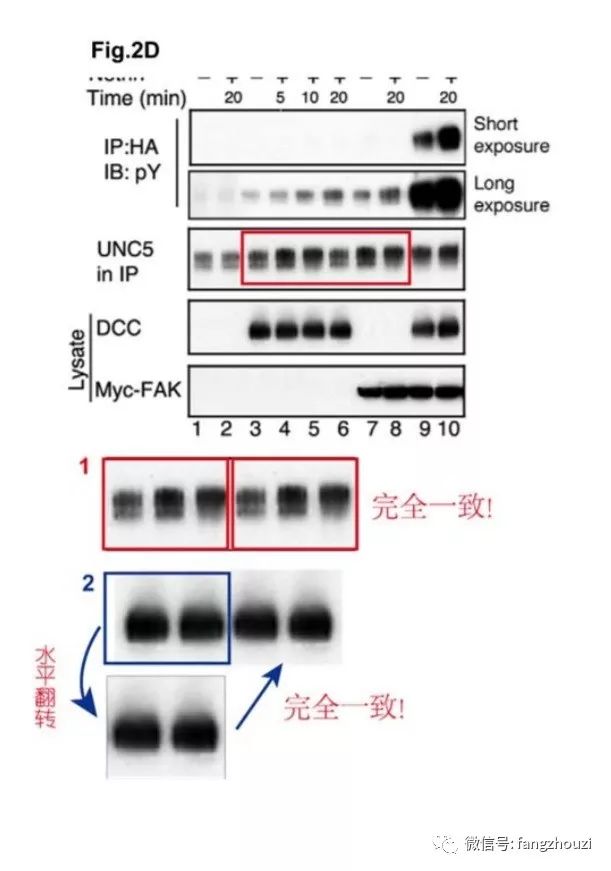
论文1是饶毅实验室为主,第一作者Guofa Liu,第二作者为管坤良实验室的Weiquan Li,还有其他研究者。现在不清楚Figure 2d and 2e具体来自哪位研究者。但问题很简单:Figure 2d and 2e 发表在959页,而描述它们的文字在961页。文字清晰地表明,它们来自同一个实验,2d显示CAS蛋白质在Y165位点的磷酸化,2e是CAS蛋白质在Y249位点的磷酸化,而它们的CAS蛋白质总量当然也是一样。
论文2是饶毅实验室与其他四个实验室合作于2004年发表的。如果结合文章读这篇文章可以知道:两个地方重复出现的图,都是实验的对照,而且重复的原因都是因为是同一个实验。如论文的1223页和1224页清晰地表明,论文2的Figure 1j 和 2c来自同一个实验,它们所含的蛋白质含量对照的样本actin当然应该一样。Figure 1j显示FAK多个磷酸化位点(397、407、576、、577、861、915)中哪几个受netrin的调节,Figure 2c显示FAK蛋白的总体磷酸化,所以它们的actin自然是一样的。
论文2的Figure 3d 和 3e :如文章1225页的文字清晰表明,它们也是同一个实验,它们所含细胞内源的Abl蛋白质作为对照也自然是一样的。
以上两篇文章出现三对一样的图,是因为每一对都来自同一个实验的对照,都不是影响结果的实验数据。如果制作演讲用的PPT,可以同一实验的同样对照用于两张PPT,方便听众看到对照实验的结果。而发表论文的图片,应该只出现一次。估计是十几年前还是学生/博士后者在制作用于实验室组会(lab meeting)的PPT之后,没有注意合并。实际上最好的办法是:1)合并论文1的Figure 2d和2e;2)合并论文2的Figure 3d和3e;3)论文2的Figure 1j可以不显示actin(因为它已显示FAK蛋白质总量的对照),只在Figure legend(图例)里面说明它的actin在2c显示。
其他图片有两篇(论文3和论文5)是管教授通讯作者,饶毅为一般作者。饶毅为这两篇文章提供的也是神经导向的结果,而不是生物化学结果,管教授看后表示全部由他实验室负责。管坤良和饶毅同为论文4通讯作者、管坤良为论文1一般作者,但论文1和论文4的中被提出问题的图也已收到Weiquan Li的回复是由他提供:论文1的 Figure 2b, 2c, 2d, 2e; 论文4的Figure 2h, 2i;和Figure 3a, 3c and 3d。Weiquan Li是论文1的第二作者、论文4的共同第一作者。
论文5有三位通讯作者,其Fig 2d(和2b、2c、2e)来自Weiquan Li,见来信。
附1:与Journal of Neuroscience杂志商定的更正说明:
Erratum: Liu et al., p130CAS Is Required for Netrin Signaling and Commissural Axon Guidance
https://doi.org/10.1523/JNEUROSCI.4616-06.2007
In the article “p130CAS Is Required for Netrin Signaling and Commissural Axon Guidance,” by Guofa Liu, Weiquan Li, Xue Gao, Xiaoling Li, Claudia Jürgensen, Hwan-Tae Park, Nah-Young Shin, Jian Yu, Ming-Liang He, Steven K. Hanks, Jane Y. Wu, Kun-Liang Guan and Yi Rao, which appeared on pages 957–968 of the January 24, 2007, issue, the authors note that the two CAS control lanes in Figure 2d were identical to the two CAS control lanes in Figure 2e: “These were control parts of the same experiment, and thus the CAS control lanes should be the same. The experimental part of Figure 2d showed CAS phosphorylation at Y165, and the experimental part of Figure 2e showed CAS phosphorylation at Y249. For figure panels in the paper, it is better to have the experimental part of Figure 2e (CAS Y249 phosphorylation lanes) combined into Figure 2d, with the CAS control lanes shown only in Figure 2d. Figure 2e can then be deleted. These changes in presentation affect no experimental results, interpretations or conclusions.”
附2:Nature Neuroscience主编回复
“Dear Yi, I would like to acknowledge receipt of your email.”
附3:管坤良致饶毅信
Dear Yi,
I am writing to state that we (my formal postdoctoral fellow Weiquan Li) provided the following figures for our collaboration papers, in which either Jane Wu or you are the primarily corresponding authors. We take full responsibility for the content of these figures. The figures contributed by us are listed below (based on the confirmation provided by Dr. Weiquan Li).
PNAS 106, 2951-2956, 2009
Figure 2b, 2c, 2d, 2e.
J. Neuroscience 27, 957-968, 2007
Figure 2h, 2i; Figure 3a, 3c, 3d.
We also take full responsibility for two other papers (Nature Neuroscience 7, 1213-1221, 2004; J Cell Sci. 119, 47-55, 2006), in which you are a co-author.
Please find the attached files with our analyses/responses. We (two current students and myself) have carefully examined the figures in the PDF files that were downloaded from the journals. We visually inspected the data when the figures were enlarged to a degree that each individual pixel was visible. In addition, we have asked for computer analysis to compare the data alleged by the anonymous accuser. Both the manual inspection and computer analysis reached similar conclusions, which are attached for your information. I would urge you or an independent person to carefully examine the figures as well.
I also attached a list of papers by other investigators that support the major conclusions of our papers.
Regards,
Kun-Liang
The following publications support the conclusions described in our paper.
Nat Neurosci. 2004 Nov;7(11):1204-12. Epub 2004 Oct 17.
Focal adhesion kinase in netrin-1 signaling.
Ren XR1, Ming GL, Xie Y, Hong Y, Sun DM, Zhao ZQ, Feng Z, Wang Q, Shim S, Chen ZF, Song HJ, Mei L, Xiong WC.
Author information
Abstract
Netrins are a family of secreted molecules that are important for axonal outgrowth and guidance in the developing nervous system. However, the signaling mechanisms that lie immediately downstream of netrin receptors remain poorly understood. Here we report that the netrin receptor DCC (deleted in colorectal cancer) interacts with the focal adhesion kinase (FAK), a kinase implicated in regulating cell adhesion and migration. FAK was expressed in developing brains and was localized with DCC in cultured neurons. Netrin-1 induced FAK and DCC tyrosine phosphorylation. Disruption of FAK signaling abolished netrin-1-induced neurite outgrowth and attractive growth cone turning. Taken together, these results indicate a new signaling mechanism for DCC, in which FAK is activated upon netrin-1 stimulation and mediates netrin-1 function; they also identify a critical role for FAK in axon navigation.
J Cell Biol. 2004 Nov 22;167(4):687-98.
Phosphorylation of DCC by Fyn mediates Netrin-1 signaling in growth cone guidance.
Meriane M1, Tcherkezian J, Webber CA, Danek EI, Triki I, McFarlane S, Bloch-Gallego E, Lamarche-Vane N.
Author information
Abstract
Netrin-1 acts as a chemoattractant molecule to guide commissural neurons (CN) toward the floor plate by interacting with the receptor deleted in colorectal cancer (DCC). The molecular mechanisms underlying Netrin-1-DCC signaling are still poorly characterized. Here, we show that DCC is phosphorylated in vivo on tyrosine residues in response to Netrin-1 stimulation of CN and that the Src family kinase inhibitors PP2 and SU6656 block both Netrin-1-dependent phosphorylation of DCC and axon outgrowth. PP2 also blocks the reorientation of Xenopus laevis retinal ganglion cells that occurs in response to Netrin-1, which suggests an essential role of the Src kinases in Netrin-1-dependent orientation. Fyn, but not Src, is able to phosphorylate the intracellular domain of DCC in vitro, and we demonstrate that Y1418 is crucial for DCC axon outgrowth function. Both DCC phosphorylation and Netrin-1-induced axon outgrowth are impaired in Fyn(-/-) CN and spinal cord explants. We propose that DCC is regulated by tyrosine phosphorylation and that Fyn is essential for the response of axons to Netrin-1.
Development. 2005 Dec;132(23):5161-72. Epub 2005 Oct 26.
SRC-1, a non-receptor type of protein tyrosine kinase, controls the direction of cell and growth cone migration in C. elegans.
Itoh B1, Hirose T, Takata N, Nishiwaki K, Koga M, Ohshima Y, Okada M.
Author information
Abstract
Src family tyrosine kinase (SFK) has been implicated in the regulation of cell adhesion and migration during animal development. We show that SRC-1, an ortholog of SFK, plays an essential role in directing cell migration in Caenorhabditis elegans. The mutation in the src-1 gene results in defective distal tip cell (DTC)-directed gonad morphogenesis in an activity-dependent and DTC cell-autonomous manners. In the src-1 mutants, DTCs fail to turn and continue their centrifugal migration along the ventral muscles. The effect of the src-1 mutation is suppressed by mutations in genes that function in the CED/Rac pathway, suggesting that SRC-1 in DTCs is an upstream regulator of a Rac pathway that controls cytoskeletal remodeling. In the src-1 mutant, the expression of unc-5/netrin receptor is normally regulated, and neither the precocious expression of UNC-5 nor the mutation in the unc-5 gene significantly affects the DTC migration defect. These data suggest that SRC-1 acts in the netrinsignaling in DTCs. The src-1 mutant also exhibits cell-autonomous defects in the migration and growth cone path-finding of Q neuroblast descendants AVM and PVM. However, these roles of SRC-1 do not appear to involve the CED/Rac pathway. These findings show that SRC-1 functions in responding to various extracellular guidance cues that direct the cell migration via disparate signaling pathways in different cell types.
Neurosignals. 2008;16(2-3):235-45. doi: 10.1159/000111566. Epub 2008 Feb 5.
Tyrosine phosphorylation of netrin receptors in netrin-1 signaling.
Ren XR1, Hong Y, Feng Z, Yang HM, Mei L, Xiong WC.
Author information
Abstract
Deleted in colorectal cancer (DCC) and neogenin are receptors of netrins, a family of guidance cues that promote axon outgrowth and guide growth cones in developing nervous system. The intracellular mechanisms of netrins, however, remain elusive. In this paper, we show that both DCC and neogenin become tyrosine phosphorylated in cortical neurons in response to netrin-1. Using a site-specific antiphosphor DCC antibody, we show that Y1420 phosphorylation is increased in netrin-1-stimulated neurons and that tyrosine-phosphorylated DCC is located in growth cones. In addition, we show that tyrosine-phosphorylated DCC selectively interacts with the Src family kinases Fyn and Lck, but not Src, c-Abl, Grb2, SHIP1, Shc, or tensin, suggesting a role of Fyn or Lck in netrin-1-DCC signaling. Of interest to note is that tyrosine-phosphorylated neogenin and uncoordinated 5 H2 (Unc5H2) not only bind to the Src homology 2 (SH2) domains of Fyn and SHP2, but also interact with the SH2 domain of SHIP1, suggesting a differential signaling between DCC and neogenin/Unc5H2. Furthermore, we demonstrate that inhibition of Src family kinase activity attenuated netrin-1-induced neurite outgrowth. Together, these results suggest a role of Src family kinases and tyrosine phosphorylation of netrin-1 receptors in regulating netrin-1 function.
Development. 2009 Feb;136(3):415-26. doi: 10.1242/dev.018234.
Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA.
Rajasekharan S1, Baker KA, Horn KE, Jarjour AA, Antel JP, Kennedy TE.
Author information
Abstract
The molecular mechanisms underlying the elaboration of branched processes during the later stages of oligodendrocyte maturation are not well understood. Here we describe a novel role for the chemotropic guidance cue netrin 1 and its receptor deleted in colorectal carcinoma (Dcc) in the remodeling of oligodendrocyte processes. Postmigratory, premyelinating oligodendrocytes express Dcc but not netrin 1, whereas mature myelinating oligodendrocytes express both. We demonstrate that netrin 1 promotes process extension by premyelinating oligodendrocytes in vitro and in vivo. Addition of netrin 1 to mature oligodendrocytes in vitro evoked a Dcc-dependent increase in process branching. Furthermore, expression of netrin 1 and Dcc by mature oligodendrocytes was required for the elaboration of myelin-like membrane sheets. Maturation of oligodendrocyte processes requires intracellular signaling mechanisms involving Fyn, focal adhesion kinase (FAK), neuronal Wiscott-Aldrich syndrome protein (N-WASP) and RhoA; however, the extracellular cues upstream of these proteins in oligodendrocytes are poorly defined. We identify a requirement for Src family kinase activity downstream of netrin-1-dependent process extension and branching. Using oligodendrocytes derived from Fyn knockout mice, we demonstrate that Fyn is essential for netrin-1-induced increases in process branching. Netrin 1 binding to Dcc on mature oligodendrocytes recruits Fyn to a complex with the Dcc intracellular domain that includes FAK and N-WASP, resulting in the inhibition of RhoA and inducing process remodeling. These findings support a novel role for netrin 1 in promoting oligodendrocyte process branching and myelin-like membrane sheet formation. These essential steps in oligodendroglial maturation facilitate the detection of target axons, a key step towards myelination.
Blood. 2010 Jul 1;115(26):5418-26. doi: 10.1182/blood-2009-11-252338. Epub 2010 Apr 20.
Netrin-4 induces lymphangiogenesis in vivo.
Larrieu-Lahargue F1, Welm AL, Thomas KR, Li DY.
Author information
Abstract
Netrin-4, a laminin-related secreted protein is an axon guidance cue recently shown essential outside of the nervous system, regulating mammary and lung morphogenesis as well as blood vascular development. Here, we show that Netrin-4, at physiologic doses, induces proliferation, migration, adhesion, tube formation and survival of human lymphatic endothelial cells in vitro comparable to well-characterized lymphangiogenic factors fibroblast growth factor-2 (FGF-2), hepatocyte growth factor (HGF), vascular endothelial growth factor-A (VEGF-A), and vascular endothelial growth factor-C (VEGF-C). Netrin-4 stimulates phosphorylation of intracellular signaling components Akt, Erk and S6, and their specific inhibition antagonizes Netrin-4-induced proliferation. Although Netrin receptors Unc5B and neogenin, are expressed by human lymphatic endothelial cells, suppression of either or both does not suppress Netrin-4-promoted in vitro effects. In vivo, Netrin-4 induces growth of lymphatic and blood vessels in the skin of transgenic mice and in breast tumors. Its overexpression in human and mouse mammary carcinoma cancer cells leads to enhanced metastasis. Finally, Netrin-4 stimulates in vitro and in vivo lymphatic permeability by activating small GTPases and Src family kinases/FAK, and down-regulating tight junction proteins. Together, these data provide evidence that Netrin-4 is a lymphangiogenic factor contributing to tumor dissemination and represents a potential target to inhibit metastasis formation.
Circ Res. 2011 Sep 16;109(7):770-4. doi: 10.1161/CIRCRESAHA.111.247239. Epub 2011 Jul 28.
Netrin-4 activates endothelial integrin {alpha}6{beta}1.
Larrieu-Lahargue F1, Welm AL, Thomas KR, Li DY.
Author information
Abstract
RATIONALE:
Netrin-4 regulates vascular development. Identity of netrin-4 endothelial receptor and its subsequent cell functions is controversial. We previously demonstrated that the inhibition of netrin-1 canonical receptors, Unc5B and neogenin, expressed by lymphatic endothelial cells, do not suppress netrin-4-induced cell signaling and functions. Netrin family members were shown to signal through a range of receptors, including integrins (such as α3β1, α6β1, and α6β4) in nonendothelial cells.
OBJECTIVE:
We tested whether integrins are netrin-4 receptors in the endothelium.
METHODS AND RESULTS:
The α6β1 integrin is expressed by endothelial cells, and binds netrin-4 in a dose-dependent manner. Inhibition of α6 or β1 integrin subunits suppresses netrin-4-induced endothelial cell migration, adhesion, and focal adhesion contact. Netrin-4-stimulated phosphorylation of Src kinase family, effectors of endothelial cell migration, is also abolished by α6 or β1 inhibition. Finally, netrin-4 and α6β1 integrin expression colocalize in mouse embryonic, intestine, and tumor vasculature.
CONCLUSIONS:
The α6β1 integrin is a netrin-4 receptor in lymphatic endothelium and consequently represents a potential target to inhibit netrin-4-induced metastatic dissemination.
Mol Cell Biol. 2013 Feb;33(4):739-51. doi: 10.1128/MCB.01264-12. Epub 2012 Dec 10.
Tyrosine phosphorylation of the Rho guanine nucleotide exchange factor Trio regulates netrin-1/DCC-mediated cortical axon outgrowth.
DeGeer J1, Boudeau J, Schmidt S, Bedford F, Lamarche-Vane N, Debant A.
Author information
Abstract
The chemotropic guidance cue netrin-1 mediates attraction of migrating axons during central nervous system development through the receptor Deleted in Colorectal Cancer (DCC). Downstream of netrin-1, activated Rho GTPases Rac1 and Cdc42 induce cytoskeletal rearrangements within the growth cone. The Rho guanine nucleotide exchange factor (GEF) Trio is essential for Rac1 activation downstream of netrin-1/DCC, but the molecular mechanisms governing Trio activity remain elusive. Here, we demonstrate that Trio is phosphorylated by Src family kinases in the embryonic rat cortex in response to netrin-1. In vitro, Trio was predominantly phosphorylated at Tyr(2622) by the Src kinase Fyn. Though the phospho-null mutant Trio(Y2622F) retained GEF activity toward Rac1, its expression impaired netrin-1-induced Rac1 activation and DCC-mediated neurite outgrowth in N1E-115 neuroblastoma cells. Trio(Y2622F) impaired netrin-1-induced axonal extension in cultured cortical neurons and was unable to colocalize with DCC in growth cones, in contrast to wild-type Trio. Furthermore, depletion of Trio in cortical neurons reduced the level of cell surface DCC in growth cones, which could be restored by expression of wild-type Trio but not Trio(Y2622F). Together, these findings demonstrate that Trio(Y2622) phosphorylation is essential for the regulation of the DCC/Trio signaling complex in cortical neurons during netrin-1-mediated axon outgrowth.
Cell Rep. 2013 Jan 31;3(1):173-85. doi: 10.1016/j.celrep.2012.12.005. Epub 2013 Jan 3.
DCC expression by neurons regulates synaptic plasticity in the adult brain.
Horn KE1, Glasgow SD, Gobert D, Bull SJ, Luk T, Girgis J, Tremblay ME, McEachern D, Bouchard JF, Haber M, Hamel E, Krimpenfort P, Murai KK, Berns A, Doucet G, Chapman CA, Ruthazer ES, Kennedy TE.
Author information
Abstract
The transmembrane protein deleted in colorectal cancer (DCC) and its ligand, netrin-1, regulate synaptogenesis during development, but their function in the mature central nervous system is unknown. Given that DCC promotes cell-cell adhesion, is expressed by neurons, and activates proteins that signal at synapses, we hypothesized that DCC expression by neurons regulates synaptic function and plasticity in the adult brain. We report that DCC is enriched in dendritic spines of pyramidal neurons in wild-type mice, and we demonstrate that selective deletion of DCC from neurons in the adult forebrain results in the loss of long-term potentiation (LTP), intact long-term depression, shorter dendritic spines, and impaired spatial and recognition memory. LTP induction requires Src activation of NMDA receptor (NMDAR) function. DCC deletion severely reduced Src activation. We demonstrate that enhancing NMDAR function or activating Src rescues LTP in the absence of DCC. We conclude that DCC activation of Src is required for NMDAR-dependent LTP and certain forms of learning and memory.
J Neurosci. 2006 Sep 20;26(38):9743-9.
Netrin-1 signaling regulates de novo protein synthesis of kappa opioid receptor by facilitating polysomal partition of its mRNA.
Tsai NP1, Bi J, Loh HH, Wei LN.
Author information
Abstract
The expression of kappa opioid receptor (KOR) is subjected to both transcriptional and posttranscriptional controls. We report that KOR translation is regulated by netrin-1 in primary neurons of dorsal root ganglion (DRG) and in P19 embryonal carcinoma cells. Without stimulation, a significant portion of KOR mRNA is maintained in a dormant state and partitions in the translationally inactive, post-polysomal fraction. During netrin-1 stimulation, which activates its downstream target focal adhesion kinase (FAK), KOR mRNA rapidly partitions to the translationally active polysomal fraction. Functionally, the newly synthesized KOR proteins in DRG neurons are able to bind to specific ligands. This report describes the first example of netrin-1 signaling in the translational control of a drug receptor KOR, which involves the mediator of netrin-1, FAK, and a novel mechanism that enhances the association of target mRNA with polysomes for translational activation.
EMBO J. 2007 Mar 21;26(6):1522-31. Epub 2007 Feb 22.
The adaptor Grb7 links netrin-1 signaling to regulation of mRNA translation.
Tsai NP1, Bi J, Wei LN.
Author information
Abstract
We previously reported a novel biological activity of Netrin-1 in translational stimulation of kappa opioid receptor (KOR). We now identify Grb7 as a new RNA-binding protein that serves as the molecular adaptor for transmitting Netrin-1 signals, through focal adhesion kinase (FAK), to the translation machinery. Grb7 binds specifically to the first stem loop of kor mRNA 5'-UTR through a new RNA-binding domain located in its amino terminus. Upon binding to its capped, target mRNA, Grb7 blocks the recruitment of eIF4E, rendering mRNA untranslatable. The RNA-binding and translation-repressive activity is reduced by FAK-mediated hyperphosphorylation on two tyrosine residues of its carboxyl terminus. This study reports an adaptor protein Grb7 that transmits the stimulating signals of Netrin-1 to the translational machinery to rapidly regulate mRNA translation.
J Cell Biol. 2009 Mar 9;184(5):737-50. doi: 10.1083/jcb.200807029.
Unc5B associates with LARG to mediate the action of repulsive guidance molecule.
Hata K1, Kaibuchi K, Inagaki S, Yamashita T.
Author information
Abstract
Neuronal axons are guided by attractive and repulsive cues in their local environment. Because the repulsive guidance molecule A (RGMa) was originally identified as an axon repellent in the visual system, diverse functions in the developing and adult central nervous system have been ascribed to it. RGMa binding to its receptor neogenin induces RhoA activation, leading to inhibitory/repulsive behavior and collapse of the neuronal growth cone. However, the precise mechanisms that regulate RhoA activation are poorly understood. In this study, we show that Unc5B, a member of the netrin receptor family, interacts with neogenin as a coreceptor for RGMa. Moreover, leukemia-associated guanine nucleotide exchange factor (LARG) associates with Unc5B to transduce the RhoA signal. Focal adhesion kinase (FAK) is involved in RGMa-induced tyrosine phosphorylation of LARG as well as RhoA activation. These findings uncover the molecular basis for diverse functions mediated by RGMa.
Mol Biol Cell. 2009 Dec;20(23):4920-31. doi: 10.1091/mbc.E09-06-0491. Epub 2009 Oct 7.
Neogenin regulates skeletal myofiber size and focal adhesion kinase and extracellular signal-regulated kinase activities in vivo and in vitro.
Bae GU1, Yang YJ, Jiang G, Hong M, Lee HJ, Tessier-Lavigne M, Kang JS, Krauss RS.
Author information
Abstract
A variety of signaling pathways participate in the development of skeletal muscle, but the extracellular cues that regulate such pathways in myofiber formation are not well understood. Neogenin is a receptor for ligands of the netrin and repulsive guidance molecule (RGM) families involved in axon guidance. We reported previously that neogenin promoted myotube formation by C2C12 myoblasts in vitro and that the related protein Cdo (also Cdon) was a potential neogenin coreceptor in myoblasts. We report here that mice homozygous for a gene-trap mutation in the Neo1 locus (encoding neogenin) develop myotomes normally but have small myofibers at embryonic day 18.5 and at 3 wk of age. Similarly, cultured myoblasts derived from such animals form smaller myotubes with fewer nuclei than myoblasts from control animals. These in vivo and in vitro defects are associated with low levels of the activated forms of focal adhesion kinase (FAK) and extracellular signal-regulated kinase (ERK), both known to be involved in myotube formation, and inefficient expression of certain muscle-specific proteins. Recombinant netrin-2 activates FAK and ERK in cultured myoblasts in a neogenin- and Cdo-dependent manner, whereas recombinant RGMc displays lesser ability to activate these kinases. Together, netrin-neogenin signaling is an important extracellular cue in regulation of myogenic differentiation and myofiber size.
J Neurosci. 2012 Aug 22;32(34):11574-85. doi: 10.1523/JNEUROSCI.0999-12.2012.
Netrin-1 attracts axons through FAK-dependent mechanotransduction.
Moore SW1, Zhang X, Lynch CD, Sheetz MP.
Author information
Abstract
The mechanism by which extracellular cues influence intracellular biochemical cascades that guide axons is important, yet poorly understood. Because of the mechanical nature of axon extension, we explored whether the physical interactions of growth cones with their guidance cues might be involved. In the context of mouse spinal commissural neuron axon attraction to netrin-1, we found that mechanical attachment of netrin-1 to the substrate was required for axon outgrowth, growth cone expansion, axon attraction and phosphorylation of focal adhesion kinase (FAK) and Crk-associated substrate (CAS). Myosin II activity was necessary for traction forces >30 pN on netrin-1. Interestingly, while these myosin II-dependent forces on netrin-1 substrates or beads were needed to increase the kinase activity and phosphorylation of FAK, they were not necessary for netrin-1 to increase CAS phosphorylation. When FAK kinase activity was inhibited, the growth cone's ability to recruit additional adhesions and to generate forces >60 pN on netrin-1 was disrupted. Together, these findings demonstrate an important role for mechanotransduction during chemoattraction to netrin-1 and that mechanical activation of FAK reinforces interactions with netrin-1 allowing greater forces to be exerted.
Mol Biol Cell. 2017 Sep 1;28(18):2374-2385. doi: 10.1091/mbc.E16-08-0594. Epub 2017 Jul 12.
TRIM9-dependent ubiquitination of DCC constrains kinase signaling, exocytosis, and axon branching.
Plooster M1, Menon S2, Winkle CC3, Urbina FL1, Monkiewicz C2, Phend KD2, Weinberg RJ2, Gupton SL4,5,6.
Author information
Abstract
Extracellular netrin-1 and its receptor deleted in colorectal cancer (DCC) promote axon branching in developing cortical neurons. Netrin-dependent morphogenesis is preceded by multimerization of DCC, activation of FAK and Src family kinases, and increases in exocytic vesicle fusion, yet how these occurrences are linked is unknown. Here we demonstrate that tripartite motif protein 9 (TRIM9)-dependent ubiquitination of DCC blocks the interaction with and phosphorylation of FAK. Upon netrin-1 stimulation TRIM9 promotes DCC multimerization, but TRIM9-dependent ubiquitination of DCC is reduced, which promotes an interaction with FAK and subsequent FAK activation. We found that inhibition of FAK activity blocks elevated frequencies of exocytosis in vitro and elevated axon branching in vitro and in vivo. Although FAK inhibition decreased soluble N-ethylmaleimide attachment protein receptor (SNARE)-mediated exocytosis, assembled SNARE complexes and vesicles adjacent to the plasma membrane increased, suggesting a novel role for FAK in the progression from assembled SNARE complexes to vesicle fusion in developing murine neurons.
J Cell Mol Med. 2017 Jan;21(1):72-80. doi: 10.1111/jcmm.12939. Epub 2016 Aug 25.
CD151 mediates netrin-1-induced angiogenesis through the Src-FAK-Paxillin pathway.
Yang X1, Li S1, Zhong J1, Zhang W1, Hua X1, Li B1, Sun H1.
Author information
Abstract
Crosstalk between the nervous and vascular systems is important during development and in response to injury, and the laminin-like axonal guidance protein netrin-1 has been studied for its involvement in angiogenesis and vascular remodelling. In this study, we examined the role of netrin-1 in angiogenesis and explored the underlying mechanisms. The effect of netrin-1 on brain tissues and endothelial cells was examined by immunohistochemistry and western blotting in a middle cerebral artery occlusion model and in human umbilical vein endothelial cells. Cell proliferation and cell cycle progression were assessed by the MTT assay and flow cytometry, and the Transwell and tube formation assays were used to examine endothelial cell motility and function. Netrin-1 up-regulated CD151 and VEGF concomitant with the activation of focal adhesion kinase (FAK), Src and Paxillin in vitro and in vivo and the induction of cell proliferation, migration and tube formation in vitro. Silencing of CD151 abolished the effects of netrin-1 on promoting cell migration and tube formation mediated by the activation of FAK/Src signalling. Netrin-1 promoted angiogenesis in vitro and in vivo by activating the FAK/Src/Paxillin signalling pathway through a mechanism dependent on the expression of the CD151 tetraspanin, suggesting the existence of a netrin-1/FAK/Src/CD151 signalling axis involved in the modulation of angiogenesis.
Oncotarget. 2016 Apr 26;7(17):24719-33. doi: 10.18632/oncotarget.8348.
Netrin-1 suppresses the MEK/ERK pathway and ITGB4 in pancreatic cancer.
An XZ1, Zhao ZG1, Luo YX1, Zhang R1, Tang XQ1, Hao D1, Zhao X1, Lv X1, Liu D1.
Author information
Abstract
The axon guidance factor netrin-1 promotes tumorigenesis in multiple types of cancers, particularly at their advanced stages. Here, we investigate whether netrin-1 is involved in the in vivo growth of pancreatic adenocarcinoma. We show that netrin-1 is significantly under-expressed in stage-I/II pancreatic ductal adenocarcinoma (PDAC). Netrin-1 over-expression effectively arrests the growth of xenografted PDAC cells without decreasing cell proliferation or increasing apoptosis in two-dimensional cultures in vitro. Integrin-beta4 (ITGB4) expression is significantly reduced, and ITGB4-knockdown mimics the tumor-suppressive effect of netrin-1, implying that ITGB4 is a main target of netrin-1 in constraining PDAC. We further show that netrin-1 signals to UNC5B/FAK to stimulate nitric oxide production, which promotes PP2A-mediated inhibition of the MEK/ERK pathway and decreases phosphorylated-c-Jun recruitment to the ITGB4 promoter. Our findings suggest that netrin-1 can suppress the growth of PDAC and provide a mechanistic insight into this suppression.
Cell Discov. 2018 Feb 20;4:8. doi: 10.1038/s41421-017-0008-8. eCollection 2018.
The binding of DCC-P3 motif and FAK-FAT domain mediates the initial step of netrin-1/DCC signaling for axon attraction.
Xu S1,2, Liu Y3,4, Li X1, Liu Y3, Meijers R5, Zhang Y3,4, Wang JH1,6.
Author information
Abstract
Netrin-1 plays a key role in axon guidance through binding to its receptor, Deleted in Colorectal Cancer (DCC). The initial step of signaling inside the cell after netrin-1/DCC ligation is the binding of DCC cytoplasmic P3 motif to focal adhesion targeting (FAT) domain of focal adhesion kinase (FAK). Here we report the crystal structure of P3/FAT complex. The helical P3 peptide interacts with a helix-swapped FAT dimer in a 2:2 ratio. Dimeric FAT binding is P3-specific and stabilized by a calcium ion. Biochemical studies showed that DCC-P3 motif and calcium ion could facilitate FAT dimerization in solution. Axon guidance assays confirm that the DCC/FAK complex is essential for netrin-1-induced chemoattraction. We propose that netrin-1/DCC engagement creates a small cluster of P3/FAT for FAK recruitment close to the cell membrane, which exerts a concerted effect with PIP2 for FAK signaling. We also compare P3/FAT binding with paxillin/FAT binding and discuss their distinct recognition specificity on a common FAT domain for axon attraction versus integrin signaling, respectively.
Oncol Rep. 2018 Oct;40(4):2325-2333. doi: 10.3892/or.2018.6614. Epub 2018 Aug 1.
Netrin-1 induces the proliferation of gastric cancer cells via the ERK/MAPK signaling pathway and FAK activation.
Yin K1, Shang M2, Dang S3, Wang L4, Xia Y5, Cui L3, Fan X3, Qu J3, Chen J3, Xu Z5.
Author information
Abstract
Netrin-1 (NTN1) has been demonstrated to promote tumorigenesis in multiple types of cancer; however, its role in the growth of gastric cancer (GC) cells has not been described in detail. In the present study, the data suggested that NTN1 knockdown significantly decreased the proliferation of GC cells, whereas NTN1 overexpression had an opposing effect. Furthermore, the use of focal adhesion kinase (FAK) inhibitor decreased the proliferation of GC cells. It was also revealed that NTN1 markedly induced the phosphorylation of FAK, extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK), but did not induce the phosphorylation of P38. In addition, the expression of ERK and JNK was markedly inhibited by treatment with FAK inhibitor. Xenograft analysis using GC cells revealed that NTN1 overexpression promoted tumor growth. Furthermore, the expression of NTN1 in samples collected from nude mice was downregulated in the NTN1 knockdown group and upregulated in the NTN1 overexpression group compared with the control short hairpin RNA group. These results suggest that NTN1-induced GC cell proliferation is mediated by activating ERK/MAPK signaling cascades via the distinct activation of FAK.
来源:方舟子、饶议科学、材料科学与工程公众号
推荐阅读:
赶快投稿吧!点击下图查看详情👇👇

让更多人了解你的成果、产品!
投稿邮箱:mse_material@163.com
商务合作请联系:微信vae6791
或者QQ1248284077
长按识别,材料行业都在关注的公众号